Introduction: The efficacy and safety of glasdegib (a selective oral inhibitor of hedgehog signaling pathway) in combination with low-dose cytarabine (LDAC) was evaluated in a randomized, phase 2 trial of newly diagnosed acute myeloid leukemia (AML) patients (BRIGHT AML 1003; NCT01546038). Patients receiving glasdegib+LDAC experienced statistically significant and meaningful gains in overall survival (OS) compared with patients receiving LDAC alone (median OS [95% CI]): 8.3 months [4.7-12.2] vs 4.3 months [1.9-5.7]). This analysis examined whether quality-adjusted survival improvements were similarly observed using a quality-adjusted time without symptoms of disease progression or toxicities (Q-TWiST) approach to evaluate possible trade-offs between time with adverse events (toxicities), time in relapse/progression (i.e., with symptoms of disease), and 'good' survival (i.e., time without toxicities or symptoms of progression [TWiST]) when comparing regimens.
Methods: OS in BRIGHT AML 1003 data, restricted to a follow-up of 20 months, was partitioned into time with toxicity (TOX: grade 3+ adverse events prior to progression), TWiST, and time post-progression (REL). Progression was defined as treatment discontinuation due to insufficient clinical response or death; patients who discontinued for other reasons (including adverse events) were censored at the date of discontinuation unless death occurred within 28 days of discontinuation. Q-TWiST was calculated by multiplying restricted mean time in each state by respective utilities (U) and then summing up the utility-adjusted time. Base case analysis used U(TOX)=U(REL)=0.5 and U(TWiST)=1.0; threshold analyses were performed varying U(TOX) and U(REL) jointly each from 0 to 1. Relative gains in Q-TWiST (i.e., Q-TWiST difference (combination vs LDAC) / OS in LDAC arm) of ≥15% were considered clearly clinically meaningful per the clinical literature. Sensitivity analysis varied the length of follow-up and AE definitions; subgroup analyses were also performed. 95% confidence intervals were obtained using the bootstrap procedure.
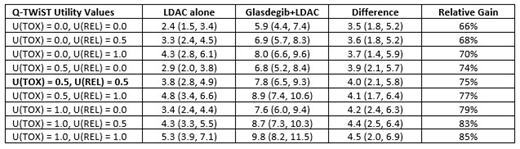
Results: At 20 months of follow-up, the survival rate for glasdegib+LDAC and LDAC arm was 28.2% and 7.9%, respectively. Glasdegib+LDAC patients (n=78) compared with LDAC patients (n=38) had significantly longer mean time in TWiST (+3.4 [95% confidence interval: 1.8, 5.2] months) and TOX (+0.8 [0.1, 1.6] months), and longer but non-significant REL (+0.3 [-1.9, 2.3] months). Q-TWiST was 4.0 [2.1, 5.8] months longer for glasdegib+LDAC, translating into a 75% relative improvement in quality-adjusted survival relative to LDAC alone. In threshold analyses, absolute and relative Q-TWiST gains ranged from 3.5 to 4.5 months and 66% to 85%, respectively (Table 1). They exceeded the clinically meaningful threshold for gains in Q-TWiST and were statistically significant across all combinations of U(TOX) and U(REL). Results were robust to length of follow-up 6 to 24 month and remained significant when including all adverse events regardless of grade.
Discussions/Conclusions: Glasdegib+LDAC is an add-on therapy that has demonstrated significant survival benefits for newly diagnosed AML patients who are unable to receive intensive chemotherapy. While patients can experience a longer time with toxicities from receiving glasdegib+LDAC (as expected since it is given as an add-on therapy), the trade-off can still be favorable as the treatment provides added time spent in 'good' health (i.e., a significantly longer time in TWiST). In the BRIGHT AML 1003 cohort, the relative gains in OS greatly exceeded previously established thresholds for being clearly clinically meaningful, which suggests that the benefits of glasdegib+LDAC vs LDAC alone outweigh the risks.
Kwon:Pfizer Inc.: Research Funding; Pharmerit International: Employment. Bell:Pfizer Inc.: Employment, Equity Ownership. Solem:Pharmerit International: Employment; Pfizer Inc.: Research Funding. Cappelleri:Pfizer: Employment, Equity Ownership. Johnson:Pfizer Inc.: Research Funding; Pharmerit International: Employment. Bhattacharyya:Pfizer Inc: Employment, Equity Ownership. Hoang:Pfizer Inc.: Employment, Equity Ownership. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Biopath Holdings: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; BiolineRx: Consultancy; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract