Translocation of t(12;22)(p13;q12) is a rare but recurrent chromosomal (chr) abnormality in hematologic malignancies involving ETS variant 6 (ETV6) and meningioma 1 (MN1) genes. The pathogenic mechanism of t(12;22)(p13;q12) and the fact that nearly half of the cases lack fusion genes remain mysterious. We focused here on AML cases with t(12;22)(p13;q12) to elucidate their molecular etiology and outcomes of allogeneic hemopoietic stem cell transplantation (allo-HSCT).
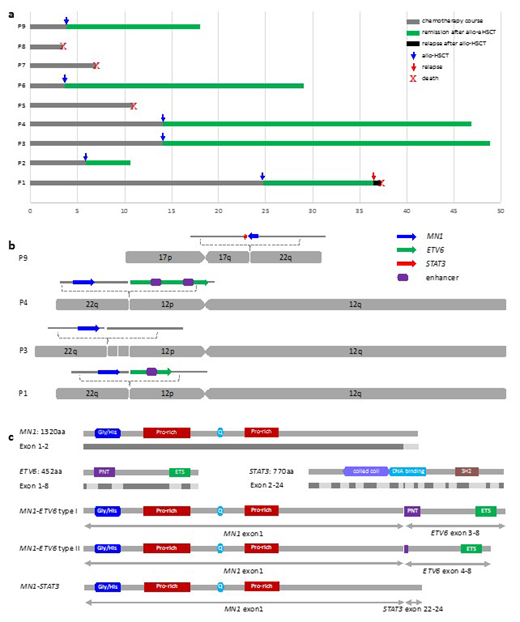
A total of eight cases with t(12;22)(p13;q12) and additionally one with t(12;17;22)( p13;q21;q13) (six males and three females) were detected in 2782 newly diagnosed AML and MDS patients by G-banding karyotyping (Table 1). The median age of onset was 45 years (range 4-60 years). The cases include three AML-M5, one AML-M2, one AML progressed from MDS (MDS-AML), one MDS with excess blasts 2 (MDS-EB-2), one chronic myelomonocytic leukemia (CMML), and two myeloid/T mixed-phenotype acute leukemia (MPAL) (Table 1). All cases underwent standard or intensive chemotherapy for myeloid malignancies, and six patients underwent allo-HSCT (Fig. 1a).
MN1-ETV6, ETV6-MN1, and MN1 expression were analyzed by RT-PCR. Mutations of 58 commonly mutated genes in hematologic malignancies were analyzed by next-generation sequencing in seven cases (Table 1). Fluorescence in situ hybridization (FISH) was performed using the MN1 dual-color break-apart probe on cases P4 and P9, who were negative for MN1-ETV6. 30 × whole-genome sequencing (WGS) was performed on diagnostic bone marrow (BM) samples from the four cases which were negative for MN1-ETV6, and structure variations including translocations (Fig. 1b) were analyzed.
5/9 cases were positive for both MN1-ETV6 and ETV6-MN1fusions (all type I), and the expression of MN1 was significantly up-regulated in 3/4 fusion negative cases (Table 1). FISH analysis showed split signals in both P4 and P9, indicating a fracture within or adjacent to MN1. WGS analysis showed ectopia of intact MN1 in case P1, P3, and P4 (Fig. 1b), including translocated adjacent to the super enhancers (SEs) of the cracked ETV6. A novel MN1-STAT3 fusion was identified and confirmed by RT-PCR in case P9.
We observed overexpression of MN1 in MN1-ETV6 negative cases in our study, which strongly indicates that the contribution of the SEs within ETV6 made on the overexpression of MN1 or aberrant activation of the chimeric MN1-ETV6. Both type I/II MN1-ETV6, and the novel MN1-STAT3 in this study retains the main functional domains of MN1 (Fig. 1c). The MN1-STAT3 contains only 69 amino acids of the STAT3 residual without functional domains, and STAT3-MN1 was absent due to the triadic translocation. Thus, the fusion protein will retain the primary function of MN1 but not STAT3 in case P9, which also indicates that it is the functionally retained MN1 within MN1-ETV6 rather than the fusion, that plays the essential role in leukemogenesis.
The genomic evidence of ETV6 disruption was observed in 8/9 cases in our study. ETV6 is a tumor suppressor which is essential in hemopoietic stem cell differentiation and multilineage blood cell development. It is conceivable that the MN1 overactivation and the ETV6 disruptive haploinsufficiency synthetically contribute to the leukemogenesis in t(12;22)(p13;q12) malignancies. This scenario is partially similar to the mechanism of GATA2-MECOM in inv(3)(q21q26) AML, within which that the central pathogenesis is the ectopia of the GATA2 SE that activates MECOM expression and confers the GATA2 haploinsufficiency simultaneously.
The present study confirmed that myeloid neoplasms with t(12;22)(p13;q12) were rare and responded poorly to conventional chemotherapy, and provide evidence that these patients can benefit from allo-HSCT. Our investigation especially highlights the evidence of a SE-associated orchestrated mechanism of MN1 overexpression and ETV6 haploinsufficiency in t(12;22)(p13;q12) myeloid neoplasms in a spatiotemporally specific manner, rather than the conventional thought of MN1-ETV6/ETV6-MN1 fusion formation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.