Introduction:
Loncastuximab tesirine (Lonca) is an antibody-drug conjugate (ADC) comprising a humanized anti-CD19 antibody (Ab) conjugated to a pyrrolobenzodiazepine dimer toxin. In a Phase 1, first-in-human ADCT-402-101 clinical study, Lonca demonstrated single-agent anti-tumor activity with manageable toxicity in patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL) and follicular lymphoma (FL). Durvalumab is a human monoclonal Ab of the immunoglobulin G-1 kappa subclass that blocks the interaction of programmed death-ligand 1 (PD-L1) with PD-1 on T-cells and with CD80 (B7.1) on other immune cells. Blockade of PD-L1/PD-1 and PD-L1/CD80 interactions releases the inhibition of immune responses, including those that may result in tumor elimination. Preclinical data, as well as early results from clinical trials combining ADCs and checkpoint inhibitors, show potentially increased effectiveness of these therapeutics when used in combination and provide the rationale for the current trial.
Study Design and Methods:
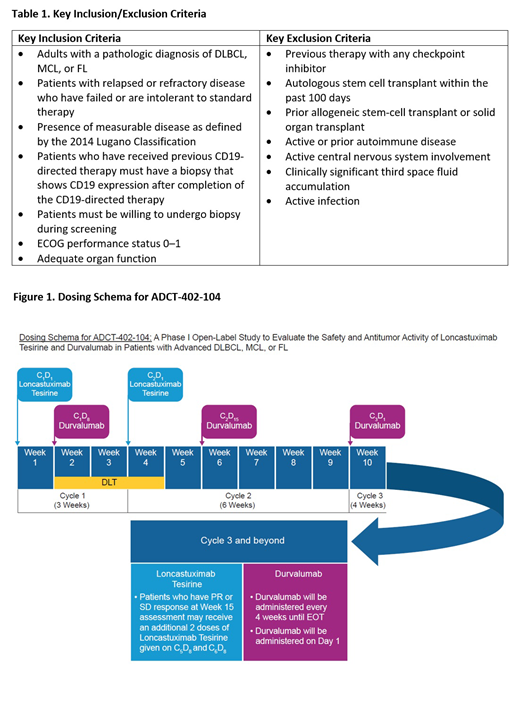
This is a Phase 1b, open-label, dose escalation (Part 1) and expansion (Part 2) trial of Lonca combined with durvalumab in patients with R/R DLBCL, MCL, or FL (NCT03685344). The key inclusion and exclusion criteria for the ADCT-402-104 study are reported in Table 1, and the dosing schema is presented in Figure 1. This trial will evaluate the safety and tolerability, preliminary anti-tumor activity, pharmacokinetics, pharmacodynamics, and immunogenicity of Lonca combined with durvalumab. Patients will receive Lonca once every 3 weeks for 2 doses in total, and durvalumab every 4 weeks for up to 1 year. Patients with only partial response or stable disease at the second disease evaluation may receive 2 additional doses of Lonca given once every 3 weeks. During Part 1, the dose of Lonca will be escalated using a classic 3+3 design with a fixed dose of durvalumab. Part 2 will consist of up to 3 expansion cohorts, one for each of the DLBCL, MCL, and FL populations. All patients in Part 2 will receive the dose of Lonca determined in Part 1, with a fixed dose of durvalumab. The trial opened in February 2019 and recruitment is ongoing.
Study sponsored by ADC Therapeutics SA with the support of MedImmune Limited, a wholly-owned subsidiary of AstraZeneca Pharmaceuticals PLC, which supplies durvalumab (http://clinicaltrials.gov/show/NCT03685344).
Moskowitz:ADC Therapeutics: Research Funding; Merck: Consultancy, Research Funding; Celgene: Consultancy; Pharmacyclics: Research Funding; Seattle Genetics, Inc.: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Ungar:ADC Therapeutics: Employment, Other: Stock options. equity interest. Dautaj:ADC Therapeutics: Employment, Other: Stock options.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract