BACKGROUND:
A risk-adapted approach incorporating genetic data, complemented by response evaluation may help to identify patients with high-risk disease (HR) who could benefit from hematopoietic stem cell transplantation (HSCT) at initial disease. Several international study groups currently recommend HSCT in pediatric HR AML. However, the impact of a risk-adapted treatment strategy is unknown. Here, we present results of our first treatment period with a risk-adapted indication for HSCT in the AML-BFM study group.
PATIENTS AND METHOD:
From 2012 until 2017 a total of 324 children <18 years of the AML-BFM registry 2012 (hereafter named R12; Germany, Austria and Czech Republic) with de novo AML were included. Down syndrome or secondary leukemia, FAB M3, an accompanying disease or pre-treatment >14 days were excluded. Patients or guardians provided written informed consent. Treatment guidelines were recommended but were not obligatory: Chemotherapy followed the best arm of study AML-BFM 2004, but patients were stratified according to new genetic and response-adapted (≥10% leukemic blasts after induction 1 or ≥5% after induction 2) risk criteria with the indication of HSCT in HR patients. We used AML-BFM 2004 (hereafter called S04) as historical comparison. The analysis was performed following the Declaration of Helsinki. Five-year estimates of overall survival (pOS) and event-free survival (pEFS) were calculated using SPSS (version 25). EFS is defined as the time from diagnosis to the first event (relapse, death, failure to achieve remission or secondary malignancy) or until last follow-up. Data were frozen on May 1st, 2019.
RESULTS:
We sought to systematically decipher the impact of a risk-adapted approach in pediatric AML.
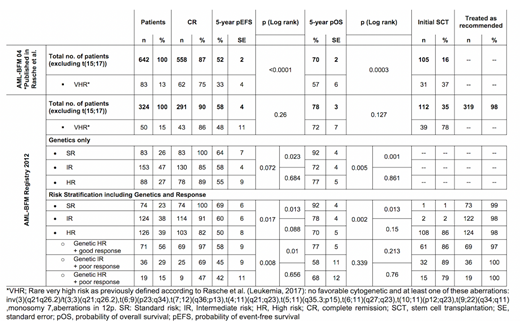
The total cohort showed a pEFS of 58±5% and pOS of 78±3% (vs S04 52±2%, p=0.014 and 70±2%, p=0.059) and significantly increased rates of HSCT (S04 vs R12: p<0.001). Importantly, the SR group did not change between periods. The increase in survival was rather explained by improvements in patients with genetically defined HR AML resulting in a survival similar to IR AML (pEFS IR vs HR: p=0.684; pOS: p=0.861). Next, we compared outcome of a previously well-defined subgroup with rare very high-risk criteria (VHR). The risk-adapted therapy resulted in a significantly higher pEFS (S04 vs R12: 33±5% vs 48±11; p=0.017) and higher rates of HSCT (37% vs 78%, p<0.001). Nevertheless, salvage treatment was equally efficient in both periods, resulting in a pOS of 56±6% vs 72±7% (p=0.202).
To evaluate the effects of inclusion of response to mere genotype-driven stratification we reanalyzed all R12 patients retrospectively according to their genetic risk only (see table 1). Response-guided re-stratification led to major shifts of patients to higher risk groups. Importantly, despite the fact that the registry made only recommendations according to the new risk stratification, compliance with guidelines including HSCT indication was very high (n=319; 98%). Discrepancies were as follows: SR have been treated with a higher intensity (i.e. more chemotherapy and/or HSCT; n=2); HR AML treated as IR (n=2), IR AML received HR treatment including HSCT (n=2). Seventy-five percent of HR AML have been transplanted. Discrepancies are explainable by early relapses or death before HSCT.
To validate the response-guided re-stratification more specifically, we performed a subgroup analysis of HR AML: The survival was similar in re-stratified IR patients and genetic HR patients with poor response (p=0.656) but was higher in genetic HR patients with good response (p=0.01), indicating an effective selection of re-stratified patients with IR.
CONCLUSION:
This analysis indicates the benefit of risk-adapted indications for HSCT in pediatric AML: After a long period with a stable pEFS (Rasche et al. Leukemia 2018) the current cohort now demonstrates a significant improvement. The efficacy of the risk-adapted approach is reflected by remarkable survival rates for patients with HR AML. At the same time, it seems not to impair the ongoing improvement of salvage therapy. However, for patients with poorly responding IR AML the outcome is dismal despite HSCT and they require alternative treatment approaches. Further studies are also needed to detect genetically defined HR patients who may not need HSCT, but also to develop efficient re-stratification approaches to enhance the survival in SR patients.
Reinhardt:Novartis: Other: Participation in Advisory Boards; Roche: Research Funding; CSL Behring: Research Funding; Jazz: Other: Participation in Advisory Boards, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.