Introduction: Obinutuzumab (GA101; G) is a fully humanized, glycoengineered, type II anti-CD20 antibody that has demonstrated substantial activity in chronic lymphocytic leukemia (CLL). Results from the primary analysis of the phase IIIb, non-randomized, open-label, single-arm GREEN safety study (NCT01905943), have previously shown that G alone or in combination with chemotherapy has a manageable toxicity profile in first-line (1L; fit and unfit) and relapsed/refractory (R/R) patients (pts) with CLL (Stilgenbauer et al. Blood 2017; Leblond et al. Haematologica 2018). Here, we report the final analysis of the GREEN study.
Methods: Enrolled pts were aged ≥18 years with documented CLL and Eastern Cooperative Oncology Group performance status 0-2. Pts received intravenous (IV) G 1000mg alone (fit or unfit pts, G-mono) on Day (D) 1, 8 and 15 of Cycle (C)1, and D1 of C2-6 (6 x 28-day cycles), with the C1D1 dose administered over 2 days, or with chemotherapy: investigator's choice of fludarabine and cyclophosphamide (G-FC) for fit pts (Cumulative Illness Rating Scale [CIRS] ≤6 and creatinine clearance [CrCl] ≥70mL/min) only; chlorambucil (G-Clb) for unfit pts (CIRS >6 and/or CrCl <70mL/min) only; or bendamustine (G-Benda) for any pt. All pts received IV corticosteroids 1h pre-dose on C1D1 and C1D2 to reduce the risk of infusion-related reactions. The primary endpoint was safety. Secondary efficacy measures included best overall response rate (BOR), complete response rate (CR), duration of response (DoR), progression-free survival (PFS), overall survival (OS), time-to-next-treatment (TTNT) and minimal residual disease (MRD). Subgroup analyses were performed on suspected prognostic biomarkers in 1L CLL. No formal statistical hypothesis tests were performed. The database lock was January 31, 2019.
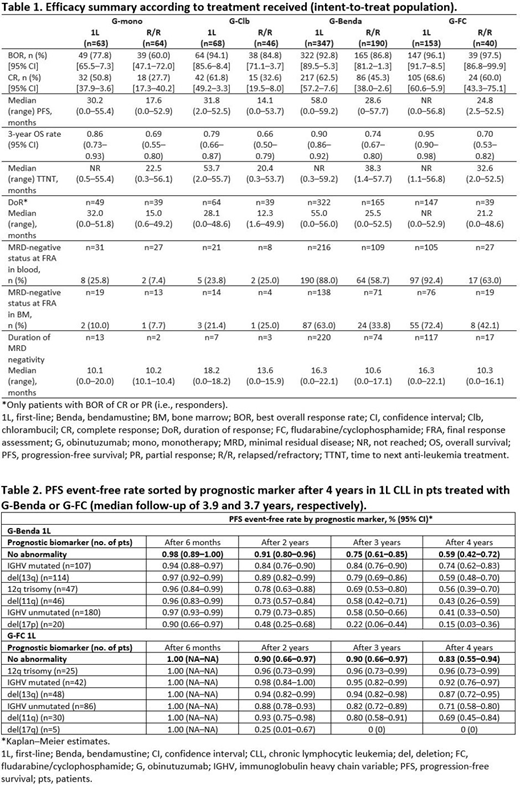
Results: Of 972 enrolled pts, 630 received 1L CLL treatment (340 fit, 290 unfit; 1 unfit pt was enrolled but was never treated), and 341 had R/R CLL. In total, 63.5% were male. The mean (range) age was 65.4 (33-90) years. Median (range) observation time was 43.7 (0.3-59.2) months. Of the 630 pts receiving 1L treatment and 341 pts who were treated for R/R disease, 488 (77.5%) and 170 (49.9%), respectively, completed the study. At final analysis, no new safety signals were observed compared with the primary safety analysis. In total, 82.7% of pts receiving 1L treatment and 84.5% of pts who were treated for R/R disease experienced grade (Gr) ≥3 adverse events (AEs), and 58.1% and 62.5% experienced serious AEs. As expected, the most common Gr 3-5 events were neutropenia (50.5% 1L, 53.4% R/R) and thrombocytopenia (14.6% 1L, 19.1% R/R). The most common non-hematological AEs were infection (any Gr: 57.6% 1L, 61.3% R/R; Gr 3-5: 21.7% 1L, 30.8% R/R) and nausea (any Gr: 27.9% 1L, 27.6% R/R; Gr 3-5: 0.8% 1L, 1.2% R/R). BOR and CR were generally higher, and DoR, PFS and TTNT were longer for pts receiving 1L therapy compared with pts who were treated for R/R disease in all arms (Table 1). Median OS was not reached at the time of analysis in any arm. The median duration of MRD-negativity was longer for pts receiving 1L therapy compared with pts who were treated for R/R disease in all arms except for pts receiving G-mono (Table 1). In addition, the MRD negative status at final response assessment in blood and bone marrow was higher for pts receiving 1L therapy compared with pts who were treated for R/R disease in all arms with the exception of pts receiving G-Clb (Table 1). Table 2 shows the PFS event-free rate by genetic marker for 1L G-Benda and G-FC over 4 years, indicating that pts with mutated immunoglobulin heavy chain variable (IGHV), del(13q) and trisomy 12 derived the most benefit.
Conclusions: In this final analysis of GREEN, no new safety signals were identified. Efficacy outcomes suggest a favorable benefit/risk profile in both 1L and R/R CLL, irrespective of chemotherapy regimen. PFS outcome for pts with 12q trisomy, del(13q) and IGHV mutated were favorable across treatment arms, while del(11q), del(17q), and unmutated IGHV showed a worse outcome. Multivariate analyses including treatment, clinical and laboratory parameters are currently being performed to identify pts who derive the most benefit.
Stilgenbauer:Pharmacyclics: Other: Travel support; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Hoffmann La-Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Bosch:AstraZeneca: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd/Genentech, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Honoraria, Research Funding; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Leblond:Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; Amgen: Honoraria, Speakers Bureau. Ilhan:Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mikuskova:Novartis: Honoraria; Roche: Honoraria; Johnson & Johnson: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; National Oncology Institute: Employment. Tausch:Roche: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Speakers Bureau. Wójtowicz:Roche: Honoraria, Other: Sponsorship of 2019 EHA participation; Acerta Pharma/AstraZeneca: Honoraria; Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Janssen: Honoraria; Takeda: Honoraria; Amgen: Consultancy. Perretti:F. Hoffmann-La Roche Ltd: Employment. Van Hoef:F. Hoffman-La Roche: Employment. Foà:Celltrion: Membership on an entity's Board of Directors or advisory committees; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Amgen Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Speakers Bureau; Shire: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
GAZYVA (obinutuzumab) is a CD20-directed cytolytic antibody and is indicated in combination with chlorambucil, for the treatment of patients with previously untreated chronic lymphocytic leukemia, and in combination with bendamustine followed by GAZYVA monotherapy, for the treatment of patients with follicular lymphoma (FL) who relapsed after, or are refractory to, a rituximab-containing regimen.
Author notes
Asterisk with author names denotes non-ASH members.