BACKGROUND
The recent approval of novel agents (NAs) has changed the treatment landscape in chronic lymphocytic leukemia (CLL), however, there remains uncertainty regarding treatment discontinuation patterns in real-world (RW) settings. This study assessed patterns of and reasons for discontinuation for patients (pts) treated with chemotherapy/chemoimmunotherapy (CT/CIT) and NAs in first (1L) and second (2L) lines of therapy in CLL.
METHODS
The CLL Collaborative Study of Real-World Evidence (CORE) study is a retrospective, multicenter, observational study. Adult CLL patients (pts) were included if they were diagnosed with CLL, initiated 1L or 2L therapy for CLL on/after 01/01/2012 (excluding lines of therapy received as part of clinical trials). Discontinuation patterns were assessed among CT/CIT and NAs, more specifically in four treatment cohorts: fludarabine+cyclophosphamide+rituximab (FCR), bendamustine+rituximab (BR), B-cell receptor inhibitors (BCRi)-based (e.g., acalabrutinib, ibrutinib, or idelalisib) and venetoclax-based regimens. Treatment discontinuation was operationally defined as ending therapy for reason(s) other than completion of planned duration of therapy.
RESULTS
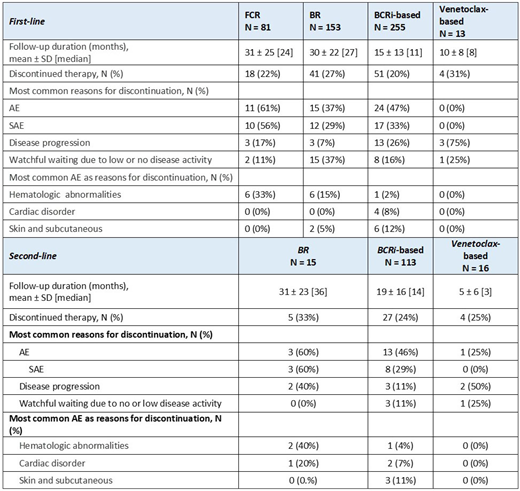
Of 671 pts receiving 1L therapy, 81 (12%) received FCR (median age=58, 70% males); 153 (23%) BR (median age=63, 61% males), 255 (38%) BCRi-based (median age=65, 65% males); and 13 (2%) venetoclax-based (median age=59, 54% males). The remaining 169 pts received other regimens (e.g., other CT/CIT). The most common BCRi-based therapy in 1L was ibrutinib-containing regimens (97%). Median duration of follow-up was 24, 27, 11 and 8 months for FCR, BR, BCRi-based and venetoclax-based regimens, respectively. Treatment discontinuation occurred in 18 (22%), 41 (27%), 51 (20%) and 4/13 pts receiving FCR, BR, BCRi-based and venetoclax-based regimens, respectively. The most common reason for discontinuation in FCR (11/18 pts; 61%), BR (15/41 pts; 37%) and BCRi-based (24/51 pts; 47%) cohorts was adverse events (AEs), with >70% being severe AEs in each cohort. Common AEs leading to discontinuations were hematological abnormalities (e.g., neutropenia, thrombocytopenia) in the FCR (6/18 pts; 33%) and BR (6/41 pts; 15%) cohorts. In the BCRi-based cohort the most common AEs leading to discontinuations were cardiac (4/51 pts; 8%), skin and subcutaneous tissue disorders (e.g., rash; 6/51 pts; 12%), hemorrhage/bleeding (3/51; 6%), and musculoskeletal and connective tissue disorders (3/51; pts; 6%). For the relatively small number of pts treated with venetoclax-based regimens and discontinued, disease progression was the common reason for discontinuations (3/4 pts); no pts discontinued venetoclax-based regimens due to adverse events.
In the relapsed/refractory setting specifically in 2L, 15 (7%), 113 (56%) and 16 (8%) pts received BR, BCRi-based and venetoclax-based regimen respectively, of which the most common BCRi-based therapy regimen was ibrutinib-based (95%). Treatment discontinuation occurred in 5/15 pts (33%), 27/113 pts (24%) and 4/16 pts (25%) receiving BR, BCRi-based and venetoclax-based regimens, respectively. The most common reason for discontinuations were severe AEs for BR (3/5 pts) and BCRi-based (13/27 pts) cohorts and disease progression for venetoclax-based regimens (2/4).
CONCLUSIONS
Despite the relatively short follow-up, in both 1L and 2L, similar discontinuation patterns emerge. CT/CIT is often discontinued prior to completion of planned cycles of therapy, suggesting that these regimens are difficult to tolerate. Additionally, treat to progression BCRi-based regimens are also discontinued for severe AEs, discordant to results from clinical trials. With a small cohort and limited information collected, results on venetoclax discontinuation warrants additional studies. Well tolerated chemotherapy-free combinations with finite treatment duration in treatment naïve and relapsed/refractory settings may limit continuous exposure to treatment and prevent discontinuations due to AEs.
Shadman:Atara: Consultancy; Gilead: Research Funding; TG Therapeutics: Research Funding; Bigene: Research Funding; Celgene: Research Funding; Acerta: Research Funding; Emergent: Research Funding; Sunesis: Research Funding; Merck: Research Funding; AbbVIe: Consultancy, Research Funding; Genentech, Inc.: Consultancy, Research Funding; AstraZeneca: Consultancy; Sound Biologics: Consultancy; Mustang Biopharma: Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy; ADC Therapeutics: Consultancy. Sail:AbbVie: Employment, Other: and may hold stock or stock options. Manzoor:AbbVie: Employment, Other: and may hold stock or stock options. Yazdy:Genentech: Research Funding; Octapharma: Consultancy; Abbvie: Consultancy; Bayer: Honoraria, Speakers Bureau. Hill:AstraZeneca: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Research Funding; Celegene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy, Honoraria; Genentech: Consultancy, Research Funding; Takeda: Research Funding; Amgen: Research Funding. Tuncer:Abbvie: Membership on an entity's Board of Directors or advisory committees; 2018 Steering Committee: Other: reimbursement for travel to the steering committee at ASH. Allan:Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie company: Consultancy; Janssen: Consultancy, Honoraria; Verastem Oncology, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Consultancy; Sunesis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy. Ujjani:Atara: Consultancy; Gilead: Consultancy; Astrazeneca: Consultancy; Genentech: Honoraria; PCYC: Research Funding; Pharmacyclics: Honoraria; AbbVie: Honoraria, Research Funding. Emechebe:AbbVie: Employment, Other: and may hold stock or stock options. Kamalakar:AbbVie: Employment, Other: and may hold stock or stock options. Sharmokh:AbbVie: Employment, Other: may hold stock or stock options. Jiang:AbbVie: Employment, Other: and may hold stock or stock options. Pena:AbbVie: Employment, Other: and may hold stock or stock options. Marshall:AbbVie: Employment, Other: and may hold stock or stock options. Nielsen:AbbVie: Employment, Other: and may hold stock or stock options. Barr:Pharmacyclics LLC, an AbbVie company: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; AbbVie: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Merck: Consultancy; Seattle Genetics: Consultancy; Genentech: Consultancy; Verastem: Consultancy; Gilead: Consultancy; Astra Zeneca: Consultancy, Research Funding. Brown:Dynamo Therapeutics: Consultancy; Catapult Therapeutics: Consultancy; Invectys: Other: other; Janssen: Honoraria; Kite: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Novartis: Consultancy; Octapharma: Consultancy; Verastem: Consultancy, Research Funding; TG Therapeutics: Consultancy; Teva: Honoraria; Sunesis: Consultancy; Pfizer: Consultancy; Pharmacyclics: Consultancy; Gilead: Consultancy, Research Funding; Genentech/Roche: Consultancy; Sun: Research Funding; Sun Pharmaceuticals, Inc: Research Funding; Morphosys: Other: Data safety monitoring boards ; Acerta Pharma: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy. Schuh:AbbVie: Consultancy, Speakers Bureau; Kite: Speakers Bureau; Gilead: Speakers Bureau; Seattle Genetics: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Janssen: Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Research Funding; Verastem: Speakers Bureau; Genentech: Consultancy, Speakers Bureau. Eyre:Janssen: Honoraria, Other: travel support; AbbVie: Honoraria, Other: travel support; Gilead: Honoraria, Other: travel support. Lamanna:Celgene: Membership on an entity's Board of Directors or advisory committees; Roche-Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bei-Gene: Research Funding; TG Therapeutics: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Wierda:Gilead Sciences: Research Funding; Juno Therapeutics: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Xencor: Research Funding; Janssen: Research Funding; Genentech: Research Funding; AbbVie: Research Funding; GSK/Novartis: Research Funding; Sunesis: Research Funding; Loxo Oncology Inc.: Research Funding; KITE pharma: Research Funding; Acerta Pharma Inc: Research Funding; Miragen: Research Funding; Pharmacyclics LLC: Research Funding; Cyclcel: Research Funding. Skarbnik:Jazz Pharmaceuticals: Speakers Bureau; Kite Pharma: Honoraria, Speakers Bureau; Novartis: Speakers Bureau; CLL Society: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Speakers Bureau; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Acerta: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem Oncology: Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Roeker:Abbott Laboratories: Equity Ownership; AbbVie: Equity Ownership. Bannerji:Celgene: Consultancy; Celgene: Consultancy; Pharmacyclics: Other: travel support; Merck: Other: travel support, Patents & Royalties: IP rights; AbbVie, Inc: Consultancy; Gilead: Other: travel support; Regeneron Pharmaceuticals, Inc.: Consultancy, Other: travel support, Research Funding; Merck: Other: travel support, Patents & Royalties: IP rights; Pharmacyclics: Other: travel support; AbbVie, Inc: Consultancy, travel support; Regeneron Pharmaceuticals, Inc.: Consultancy, Other: travel support, Research Funding; Gilead: Other: travel support. Pauff:AbbVie: Employment, Other: and may hold stock or stock options. Schuster:Novartis: Other: a patent (with royalties paid to Novartis) on combination therapies of CAR and PD-1 inhibitors.; Novartis, Celgene, Genentech, Merck, Pharmacyclics, Acerta, and Gilead: Other: Grants, Research Funding; Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, Acerta, and Celgene: Honoraria; Novartis, Nordic Nanovector, and Pfizer: Membership on an entity's Board of Directors or advisory committees. Follows:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Cheson:Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Symbios: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trillium: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Research Funding; Bristol Myers Squibb: Research Funding; Portola: Research Funding; Kite: Research Funding; Gilead: Research Funding; Epizyme: Research Funding; Morphosys: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Acerta: Consultancy, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Eichhorst:BeiGene: Research Funding; ArQule: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Brander:DTRM Biopharma: Research Funding; BeiGene: Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy; Genentech: Consultancy, Honoraria, Research Funding; Teva: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Research Funding; Novartis: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; MEI: Research Funding; Tolero: Research Funding; Acerta: Research Funding. Pivneva:AbbVie: Other: employee of Analysis Group, Inc., which has received consultancy fees from AbbVie. Guerin:AbbVie: Other: employee of Analysis Group, Inc., which has received consultancy fees from AbbVie. Mato:AstraZeneca: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Celgene: Consultancy; Johnson & Johnson: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Gilead: Research Funding; Acerta: Consultancy; Janssen: Consultancy; LOXO: Consultancy, Research Funding; DTRM Biopharma: Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB member, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.