Background:
[18F]-Fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) positivity after first line treatment with autologous stem cell transplantation (ASCT), is strongly correlated with reduced progression free survival and overall survival (Moreau et al., JCO, 2017). However, FDG PET/CT positive patients who obtain FDG PET/CT negativity after treatment can have comparable outcomes to patients who were FDG PET/CT negative at baseline (Davies et al., Haematologica 2018). Aiming for FDG PET/CT negativity may therefore be an important goal in myeloma treatment. The use of FDG PET/CT positivity as an indication for consolidation therapy after ASCT has not been studied before.
Methods:
This is an ongoing, multicenter phase II study. Patients with multiple myeloma who have received standard first line treatment including ASCT and achieved very good partial response (VGPR) or better, are eligible for the study and examined by FDG PET/CT. Patients who are FDG PET/CT positive defined by the Italian Myeloma criteria for PET USe (IMPETUS) (Nanni C et al., EJNMMI 2016 and 2018) are included in the treatment phase of the study and are assessed for minimal residual disease (MRD) by Euroflow (sensitivity: 10-5) before treatment. The treatment consists of four 28-day cycles of KRd (carfilzomib 36 mg/m2 day 1,2,8,19,15 and 16 (except 20 mg/m2 day 1 and 2 first cycle), lenalidomide 25 mg day 1-21 all cycles and dexamethasone 40 mg day 1,8,15 and 22 all cycles). After four cycles, FDG PET/CT and Euroflow for MRD are repeated for response evaluation. Both patients with FDG PET/CT negativity and patients with FDG PET/CT positivity at baseline are followed for progression free survival (PFS) and overall survival (OS).
Results:
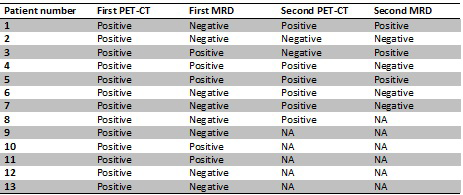
As of 1st of July 2019, 43 patients have been screened in the study. Sixteen patients (37%) had a positive FDG PET/CT result. Eight of 13 (62%) patients with a FDG PET/CT positive result were MRD negative. Eight patients have completed four cycles of KRD consolidation; two patients were converted into FDG PET/CT negativity; one of the two remained MRD positive, the other remained MRD negative. Three patients had reduced FDG uptake but were still considered FDG PET/CT positive; one of these converted from MRD positive to negative. One had stable disease and two had progression on FDG PET/CT whereof one converted from MRD negative to positive.
Conclusion:
A significant proportion (37%) of patients treated with standard first line treatment including ASCT with very good partial response or better was considered FDG PET/CT positive. Sixty-two percent of these patients were MRD negative by Euroflow, confirming the complementary features of these two methods. Treatment with four cycles of KRd improved disease status based on FDG PET/CT in 5 of 8 patients (62,5%) and converted 2 out of 8 patients to FDG PET/CT negativity. One patient was converted from MRD positivity to MRD negativity. This study is ongoing and will enroll 50 FDG PET/CT positive patients. Before the ASH 2019 meeting, we plan to screen approximately fifteen additional patients with FDG PET/CT and about five more patients will have completed KRd consolidation therapy.
Nørgaard:Bayer, Astrazeneca: Honoraria. Abildgaard:Janssen: Research Funding; Celgene: Research Funding; Takeda: Research Funding; Amgen: Research Funding. Revheim:South-Eastern Norway Regional Healt Authority: Research Funding. Schjesvold:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; SkyliteDX: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.