BACKGROUND: Acute graft versus host disease (aGVHD) represents a serious and potentially life-threatening condition in patients receiving hematopoietic stem cell transplantation (HSCT). Development of aGVHD is characterized by increased levels of inflammatory mediators and activated T cells in circulation, leading to tissue and organ damage. Previously, we demonstrated that a panel measuring ST2, REG3A, and TNFR1 poorly predicted response to the combination of corticosteroids and itacitinib, a potent and highly selective JAK1 inhibitor, in a parallel-cohort phase 1 trial (NCT02614612). In this study, we utilized broad proteomic analysis to identify potentially predictive biomarkers of therapeutic response to the combination treatment.
METHODS: Plasma samples were collected from 25 patients enrolled in the Phase 1 clinical trial prior to and at designated times following treatment. Broad proteomic analysis was conducted by OLINK Proteomics using a proximity extension assay. Selected biomarkers were quantitated by the same method based on standard curves generated using recombinant proteins. Statistical differences were identified using unpaired T tests and significance conferred when p<0.05. All participants provided written consent before enrollment.
RESULTS: The 25 patients in this study were stratified based on overall response to treatment with itacitinib and corticosteroid at day 28 (based on CIBMTR). Patients included 10 complete responders (CR), 1 very good partial responder (VGPR), 8 partial responders (PR) and 6 patients with progressive disease and/or death (PD/death) prior to day 28.
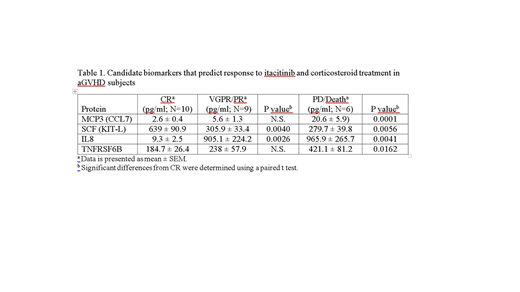
Novel biomarkers were identified and associated with therapeutic response by comparing the levels of approximately 1000 proteins in the CR (N=10) and PD/Death (N=6) cohorts specifically. The initial analysis identified 50 proteins significantly upregulated (P<.05) and 52 proteins significantly down-regulated (P<.05) in the CR cohort at baseline compared with the PD/Death cohort. The list of candidates were further screened based on correlation to known aGVHD biomarkers, degree of separation between the populations, as well as reliable and consistent testing (ie, coefficient of variation <20%). From these analyses, candidate biomarkers were identified and representative examples are shown in table 1.
Novel biomarkers include macrophage chemotactic protein 3 (MCP3/CCL7), stem cell factor (SCF/KIT-L), interleukin 8 (IL8), and tumor necrosis factor receptor super family 6B (TNFRSF6B). Each of these candidate biomarkers demonstrated a significant (P<.05) difference between CR and PD/death cohorts, with intermediate levels detected in patients with an intermediate (VGPR/PR) response to treatment. Because MCP3, IL8, and TNFRSF6B presumably associate with inflammation, elevation of these biomarkers in the PD/Death cohort that poorly respond to a JAK inhibitor (JAKi) is not surprising. In addition, elevation of SCF in responders to JAKi is consistent with supporting hematopoiesis.
CONCLUSION: Potentially novel biomarkers may be useful in predicting complete responses to treatment with a combination of corticosteroids and itacitinib in patients with aGVHD post HSCT.
Pratta:Incyte Research Institute: Employment. Liu:Incyte: Employment, Equity Ownership. Owens:Incyte Corporation: Employment, Equity Ownership. Yan:Incyte Corporation: Employment. Arbushites:Incyte: Employment. Howell:Incyte: Employment.
Author notes
Asterisk with author names denotes non-ASH members.