Background: Evomela, a Melphalan bioequivalent, was approved by the FDA in 2016 for high-dose conditioning treatment prior to hematopoietic stem cell transplantation for multiple myeloma (MM). Evomela has increased solubility and stability compared to traditional Melphalan which requires propylene glycol, a stabilizing agent. A retrospective review (Miller et al. 2019) showed that there was no difference in outcomes or short term morbidity in autologous stem cell transplant (ASCT) recipients conditioned with either Melphalan or Evomela. There was, however, an increased incidence of C. difficile-negative diarrhea in the Evomela group. Engraftment syndrome (ES) is a well characterized, although poorly understood, conglomerate of symptoms occurring in the autologous peri-engraftment period. We have previously demonstrated (McKiernan et al. 2017) that patients with ES have an adverse overall outcome. This study aims to evaluate the effect of Evomela conditioning on patients with MM receiving ASCT.
Methods: Our study cohort included 644 patients with MM who received ASCT between January 2008 and December 2018. Evomela conditioning was administered to all patients treated on or after September 4, 2016, defining the Melphalan and Evomela cohorts. ES was defined as diarrhea, rash, non-infectious fever, hepatic dysfunction, pulmonary infiltrates, or encephalopathy not attributed to other causes from 3 days prior to 15 days post engraftment. High-risk disease (HRD) was defined as del 17p, 1q gain, t(4;14), t(14;16), t(14;20) by FISH, monosomy 13, del 13q or hypodiploidy by standard cytogenetics, or high-risk gene expression profiling. Response criteria from the International Myeloma Working Group was used to determine response. Progression free survival (PFS) and overall survival (OS) probabilities were estimated using log rank or Wilcoxon tests. Cox hazard regression model was examined for factors influencing ES.
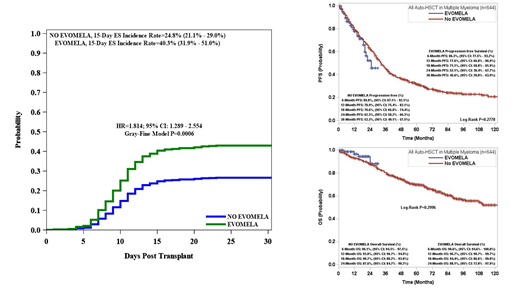
Results: Of the 644 patients, 78 were conditioned with Evomela and 554 were conditioned with Melphalan. Thirty five percent of the total patient population had HRD, 234 (36%) were age 65 or older, and 369 (57%) were males. A total of 197 (30%) patients developed ES with 171 (87%) receiving treatment with corticosteroids. Conditioning with Evomela was associated with a significantly higher incidence of ES 15 days post ASCT compared to Melphalan (40.3% vs 24.8%, p=0.0006). Multivariate analysis showed that patients conditioned with Evomela were 60% more likely (HR-1.597, 95% CI, 1.116-2.285, p=0.0105) to develop ES than traditional Melphalan. Across both cohorts, higher median CD34+ stem cell doses (5.22 vs 5.85 x 10e6/kg, p=0.0026) were protective against ES. Age greater than 65 was associated with increased 15 day post ASCT incidence of ES (HR-1.903, 95% CI, 1.435-2.523, p=<0.0001). There was no PFS (p=0.2996) or OS (p=0.2778) difference between the Evomela group and the Melphalan group. There was a trend towards decreased OS (p=0.0914) among patients with ES, but it was not statistically significant. There was no statistically significant progression difference between ES and non-ES groups (p=0.9739).
Conclusion: Patients conditioned with Evomela are significantly more likely to develop ES than patients conditioned with traditional Melphalan. We were not able to show any survival or progression-free survival advantage for patients treated with Evomela. We would caution the use of Evomela in patients with other risk factors for ES. More studies are needed to further understand the differences between Melphalan and Evomela.
Siegel:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Rowley:Allergan: Equity Ownership; Fate Therapeutics: Consultancy. Biran:Merck: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Bristol Meyers Squibb: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Goldberg:Bristol-Myers Squibb: Consultancy; COTA: Equity Ownership; Cancer Outcomes Tracking and Analysis (COTA) Inc.: Equity Ownership. Goy:Hackensack University Medical Center, RCCA: Employment; Takeda: Other: Grants outside of the submitted work; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: leadership role for profit healthcare company; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astrazenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Other: Grants outside of the submitted work, Research Funding; Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Pharmacyclics/Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; University of Nebraska: Research Funding; Hakensackumc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.