Background: Patients with chronic liver disease (CLD) often have severe thrombocytopenia (platelet counts <50,000/mL) that can complicate the invasive diagnostic and therapeutic procedures these patients require as part of their clinical management, due to the increased bleeding risk. Avatrombopag is a thrombopoietin receptor agonist (TPO-RA) that is approved for the treatment of thrombocytopenia in patients with CLD as an alternative to platelet transfusions for patients undergoing a procedure. The aim of this study was to evaluate the relative cost-effectiveness of avatrombopag compared with platelet transfusion or treatment with lusutrombopag, another TPO-RA also approved for the treatment of thrombocytopenia in adult patients with CLD.
Methods: A decision-tree model was developed from a US payer perspective to capture the clinical events observed in registration trials, and to project potential longer-term complications resulting from a major bleed or thromboembolic event in the scenario analyses. Treatment costs were taken from publicly available data sources; avatrombopag and lusutrombopag estimates were calculated from the US prescribing information and Phase 3 study data. The interventions were evaluated in the overall trial population, patients with platelet counts <50,000/mL, and in subpopulations with higher (>40,000/mL to <50,000/mL) and lower (<40,000/mL) Baseline platelet counts. The primary metric for this economic analysis was the per-person total cost, the cost of prophylactic platelet transfusions required, and the incremental cost per prophylactic platelet transfusion avoided.
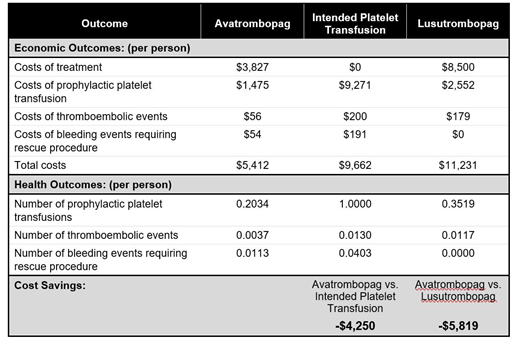
Results: In the overall population, avatrombopag reduced the need for platelet transfusions and produced cost-savings per person compared to Intended Platelet Transfusion (80% fewer prophylactic platelet transfusions), resulting in a relative cost savings of $4,250. The cost for lusutrombopag (15% more platelet transfusions) relative to avatrombopag was $5,819 higher than the cost of avatrombopag. Similar results were seen in both the higher and lower platelet count subgroups. The one-way and probabilistic sensitivity analyses showed that the use of avatrombopag remained cost-saving over a wide range of changes in input variables, with the incremental cost-effectiveness ratio falling into quadrant IV (decreased costs while prophylactic platelet transfusions were avoided).
Conclusions: From the cost-effectiveness standpoint, the use of avatrombopag is a practical strategy compared with the cost of both platelet transfusion and lusutrombopag, as it saves costs and reduces the need for prophylactic platelet transfusions.
Allen:Dova Pharmaceuticals: Equity Ownership, Other: Chief Medical Officer . Aggarwal:Dova Pharmaceuticals: Employment. Vredenburg:Dova Pharmaceuticals: Employment, Other: Shareholder.
Author notes
Asterisk with author names denotes non-ASH members.