Background: Pyruvate kinase (PK) deficiency is an under-recognized hereditary disease that causes lifelong hemolytic anemia. Mutations in the PKLR gene lead to reduced red cell PK (PK-R) enzyme activity, resulting in defective glycolysis and decreased lifespan of red blood cells. Mitapivat (AG-348) is a novel, first-in-class, oral, small-molecule allosteric activator of PK-R under clinical testing as the first targeted, disease-altering therapy for PK deficiency. In the DRIVE PK study (NCT02476916; a phase 2, open-label, dose-ranging trial in adults with PK deficiency who are not regularly transfused), twice-daily (BID) dosing with mitapivat for >6 months was well tolerated and induced rapid, durable responses (Grace et al. ASH 2017). As of July 14, 2017, 26 (50%) of 52 enrolled subjects had a maximum hemoglobin (Hb) increase of >1 g/dL. Among these 26 subjects, the mean maximum Hb increase was 3.4 g/dL, and 25 (96%) had ≥1 missense PKLR mutation. Based on these findings, mitapivat has entered phase 3 clinical development. The design of the ongoing phase 3 ACTIVATE-T study and its extension study are reported here, with an update on country/site activation and key early learnings on trial logistics in PK deficiency.
Methods: ACTIVATE-T is a global, multicenter, open-label study (NCT03559699) to evaluate the efficacy and safety of mitapivat in regularly transfused adults with PK deficiency. Regularly transfused is defined as ≥6 transfusion episodes in the previous year. Subjects who are homozygous for the R479H mutation or have 2 non-missense mutations (without the presence of another missense mutation) in PKLR will be excluded, as will subjects with an average transfusion frequency of more than once every 3 weeks in the previous year.
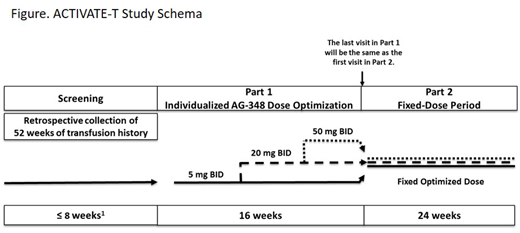
The study comprises an 8-week screening period, during which each subject's complete transfusion history from the prior 52 weeks is documented, followed by a 16-week dose optimization period and a 24-week fixed-dose period (Figure). During the dose optimization period, each subject will undergo individualized mitapivat dose optimization. All subjects will start on a dose of 5 mg BID, which may be increased twice over the course of 16 weeks (from 5 to 20 mg BID and from 20 to 50 mg BID). In the fixed-dose period, each subject will receive mitapivat at their optimized dose for 24 weeks. During the study, subjects will be transfused when their Hb reaches or falls below their individual transfusion trigger calculated from their transfusion history. The primary endpoint is the proportion of subjects who achieve a reduction in transfusion burden, defined as a reduction of ≥33% in the number of red blood cell units transfused during the 24 weeks of the fixed-dose period compared with the historical transfusion burden standardized to 24 weeks. Secondary endpoints include safety.
All subjects who complete the study will have the opportunity to enroll in an open-label extension study (NCT03853798) in which all participants will receive mitapivat for up to 192 weeks. The ACTIVATE-T trial is enrolling globally.
Lynch:Agios: Employment, Equity Ownership. Hua:Agios Pharmaceuticals, Inc.: Employment, Equity Ownership. Mix:Agios: Employment, Equity Ownership. Porter:Bluebird bio: Consultancy, Honoraria; Silence therapeutics: Honoraria; Vifor: Honoraria; La Jolla: Honoraria; Protagonism: Honoraria; Celgene: Consultancy, Honoraria; Agios: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.