Background
Patients receiving the BTK inhibitor ibrutinib (ibr) for CLL rarely achieve CR with undetectable MRD (U-MRD) and require indefinite suppressive therapy. This results in a cumulative risk of relapse and also of adverse events leading to drug discontinuation. The risk of relapse is highest in patients with complex karyotype and/or del(17p); some series also suggest increased risk in patients with del(11q) or a persistently elevated β2-microglobulin.
The Bcl-2 inhibitor venetoclax (ven) shows in vitro synergy with ibr. The combination has been well-tolerated and highly effective in first-line and relapsed/refractory patients with CLL.
Methods
We designed a phase II, investigator-initiated, response-adapted clinical trial with the addition of ven to ibr in patients (pts) with high-risk disease. Venetoclax (being co-developed by AbbVie and Genentech) and study funding were provided by AbbVie. Pts were eligible if they had received at least 1 year of ibrutinib therapy (either as first line therapy or for relapsed/refractory disease), had detectable disease without meeting IWCLL criteria for progression and had one or more high risk features for disease progression: del(17p); complex karyotype; del(11q); elevated β2-microglobulin; TP53 mutation. Ibr was continued at 140-420mg/d and standard, weekly dose-escalation of ven was performed, beginning at 20mg/d, until a target dose of 400mg/d was reached. Treatment with the combination of ibr and ven could continue for up to 2 years. Pts had bone marrow evaluation for MRD using standard 4 color flow cytometry (sensitivity 10-4) and CT scan for re-staging every 6 months; patients in CR with U-MRD on two consecutive evaluations stopped ven, but could continue ibr at treating physician discretion. Pts who are not in CR or are MRD+ at final re-staging will continue ibrutinib maintenance. The primary endpoint is achievement of U-MRD after 12 months of combination therapy.
Results:
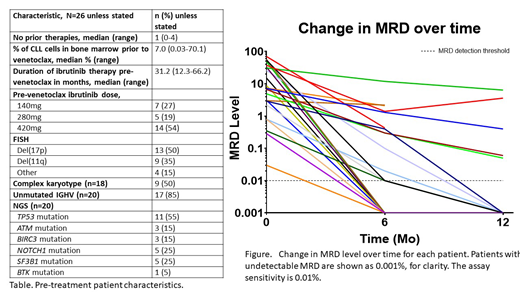
Thirty-five pts have enrolled thus far; we report results from the first 26 pts. Pre-treatment characteristics are shown in the table.
Treatment has been well-tolerated. There was no tumor lysis syndrome. Adverse events occurring in at least 20% of pts, regardless of attribution, were: diarrhea (n=14, 54%), neutropenia (n=9, 35%), nausea (n=8, 31%), fatigue (n=8, 31%). Grade 3 adverse events occurred in 11/26 pts (neutropenia, n=3, thrombocytopenia, n=2, cholecystitis, diarrhea, hypertension, pyelonephritis, skin abscess, chilblains, metastatic melanoma all n=1). Grade 4 neutropenia occurred in 1 pt and grade 4 metastatic mucinous adenocarcinoma of the lung in 1 pt. There were 14 SAEs: 6 localized non-melanoma skin cancers (NMSCs) - 5/6 had had NMSCs removed prior to ven treatment; 1 metastatic melanoma; 1 localized bladder TCC; 1 metastatic mucinous adenocarcinoma of the lung; 1 episode of pyelonephritis; 1 skin abscess; 1 inadvertent overdose of ibr without clinical consequences; 1 episode of cholecystitis. None of the other cancers were felt to be treatment related. Ven was permanently dose-reduced in 8/26 patients (to 300mg in 2 pts, 200mg in 5 pts and 100mg in 1 pt), most commonly due to neutropenia (n=5) or diarrhea (n=3) Ibr was permanently dose-reduced in 7/26 pts (to 280mg in 4 and 140mg in 3).
Three pts have discontinued therapy (2 prior to first response assessment): 1 was found to be ineligible soon after commencing treatment; two developed metastatic solid tumors (melanoma, n=1, lung cancer, n=1) requiring systemic therapy. No pt has stopped treatment due to toxicity, no pt has developed CLL progression or Richter Transformation and no pt has died while on study.
U-MRD in BM was achieved in 8/24 pts (33%) at 6 months and in 10/15 (67%) at 12 months, Figure. No patient was in CR prior to ven. At 6 months, 9/24 (38%) had achieved CR and 7/15 (47%) at 12 months. Two pts have stopped treatment after achieving CR with U-MRD at the 6 and 12 month evaluation. Twenty-one pts continue on therapy.
Conclusions:
Ven added to ibr in pts with high-risk CLL as consolidation is well tolerated and associated with a high likelihood of achieving U-MRD in BM and CR within 12 months of combination therapy, suggesting that time-limited therapy may be feasible in high-risk CLL. Further follow-up will determine the likelihood of achieving U-MRD at later time points and durability of responses.
Thompson:Genentech: Consultancy, Honoraria; Pharmacyclics: Research Funding; Pfizer: Research Funding; Amgen: Consultancy, Research Funding; AbbVie: Research Funding; Gilead: Consultancy, Honoraria. Jain:Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding. Kadia:BMS: Research Funding; AbbVie: Consultancy, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Bose:Kartos: Consultancy, Research Funding; Incyte Corporation: Consultancy, Research Funding, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding; Blueprint Medicine Corporation: Consultancy, Research Funding; Constellation: Research Funding; Pfizer: Research Funding; Astellas: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; CTI BioPharma: Research Funding. Wierda:Loxo Oncology Inc.: Research Funding; Acerta Pharma Inc: Research Funding; Janssen: Research Funding; Juno Therapeutics: Research Funding; AbbVie: Research Funding; Genentech: Research Funding; Oncternal Therapeutics Inc.: Research Funding; Miragen: Research Funding; Xencor: Research Funding; Gilead Sciences: Research Funding; GSK/Novartis: Research Funding; Pharmacyclics LLC: Research Funding; Sunesis: Research Funding; KITE pharma: Research Funding; Cyclcel: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.