Gaucher disease type 1 is the most common inherited lysosomal storage disorder caused by the deficiency of lysosomal β-glucocerebrosidase (GBA, acid-β-glucosidase), required for the degradation of glycosphingolipids. The deficiency of the enzyme results in the widespread accumulation of glucosylceramide in macrophages, leading to anemia, thrombocytopenia and coagulopathy, visceral (hepatosplenomegaly, lungs) and skeletal manifestations (deformities, fractures, avascular osteonecrosis). However, GD manifestations are caused not only by the burden of glucosylceramide storage, but also by macrophage activation. It seems that GD reflects the downstream consequences of inappropriate macrophage activation, with the release of pro-inflammatory cytokines and other responses to storage material.
The aim of this study is to assess in vitro phenotypic characterization, functional properties and gene expression anaysis of GD1 macrophages in order to understand their possible role in inflammation and in impairment of iron metabolism occurring in GD patients.
Monocytes were isolated from buffy coats of GD patients (n=3) and controls (n=3) by applying an in vitro model protocol. Monocytes were expanded in ImmunoCult™ medium and 1% ZellShield® antibiotic cocktail. Differentiation was induced in ImmunoCult™ medium, 50 ng/mL M-CSF and 50 ng/mL GM-CSF. To mimic the in vivo condition, macrophage population was loaded with erythrocytes ghosts isolated from GD patients. Cell morphology was analyzed on cytocentrifuged preparations stained with May-Grünwald Giemsa (MGG). Surface marker expression (CD11, CD33, CD68, CD64) were examined by flow cytometry to evaluate macrophages differentiation and phenotype. Gene expression analysis of iron metabolism-related genes was evaluated through Real-Time Quantitative PCR. Biochemical indices (NTBI, GDF15, sTfR and chitotriosidase) were analyzed in supernatant through ELISA assay.
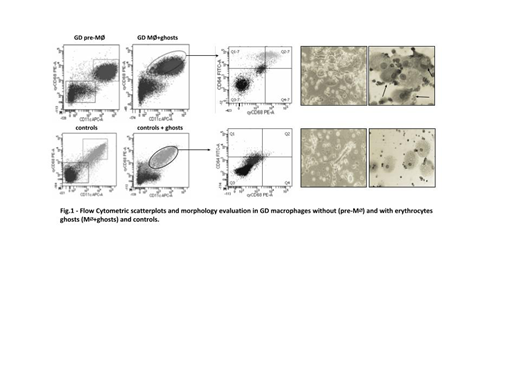
Flow cytometry analysis (Fig.1) revealed that without erythrocytes ghosts (preM∅), the proportion of CD11+/CD68+ macrophage population was similar between GD patients and control. However, in GD macrophages, when loaded with Gaucher erythrocytes ghosts (M∅+ghosts), the proportion of CD11++/CD68++ cells increased (36.4%), as a reflection of a more pro-inflammatory phenotype. Treated controls showed no differences. To further characterize these different macrophages subpopulations in GD patients, we used an additional parameter, CD64, because CD64/CD68 markers are specific for M1/M2 polarization. GD M∅+ghosts showed an higher percentage of CD64++/CD68++ population (78%) in comparison of GD pre-M∅ (45%) and controls (36%), confirming a M1 proinflammatory phenotype of GD macrophages loaded with erythrocytes ghosts. Under microscope evaluation, GD M∅+ghosts presented high number of spindle-shaped fibroblastoid, typically M1 phenotype cells, rather than large flat-round cells (M2 cells). Moreover, morphological staining of these cells confirmed the typical features of GD cells with basophilic cytoplasm with characteristic crinkles. Controls showed no differences in macrophage features when loaded with erythrocytes ghosts. To confirm the pro-inflammatory potential of GD M∅+ghosts, high levels of pro-inflammatory mediators TNF-α, IL-1β and (s)TfR) were found in supernatant of GD M∅+ghosts (72,9±8,5, 24,7±2,7 and 3,1±4,4, respectively) compared to pre-M∅ and controls. High GDF15 expression in GD M∅+ghosts (14,77±5,87) respect to preM∅ (1,80±1,1) and controls (1,67±0,8) was observed. By gene expression analysis we observed in GD M∅+ghosts an higher HAMP expression (28,13±5,63) compared to preM∅ (2,49±1,5) and controls (0,71±0,02), and a lower SLC40A1 expression (0,01±0,00) compared to GD preM∅ (0,16±0,05) and controls (0,79±0,25). No significant differences in TFRC and GDF11 expression between GD preM∅ and M∅+ghosts in GD patients were observed.
These preliminary data suggest that GD macrophages, when stimulated, display a proinflammatory potential. These activated M1 macrophages could contribute to an inflammatory-producing environment, triggering hepcidin and ferroportin expression by an autocrine/paracrine mechanism and leading to dysregulation of iron distribution.
Cappellini:CRISPR Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vifor Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria; Genzyme/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees. Motta:Sanofi-Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.