Background: Venous thromboembolism (VTE) occurs frequently in cancer patients and is commonly treated with therapeutic anticoagulation. Treatment of VTE in cancer patients with brain metastasis is an area of concern due to known risk of intracerebral hemorrhage (ICH). We evaluated risk of ICH in cancer patients with known brain metastases with or without anticoagulation.
Methods: Cancer patients diagnosed with brain metastasis between 12/2005 and 7/2018 were identified using a radiation oncology database and electronic records were retrospectively reviewed. Patients with primary brain tumors and absence of follow-up brain imaging were excluded. Clinically significant ICH was defined as ICH resulting in focal neurologic deficit or requiring neurosurgery. Overall survival (OS) calculated from initial diagnosis of brain metastasis, estimated by the Kaplan-Meier method, and compared by the log-rank test. Predictors identified as statistically significant on univariate logistic regression analysis (p<0.05) were selected for multivariate analysis.
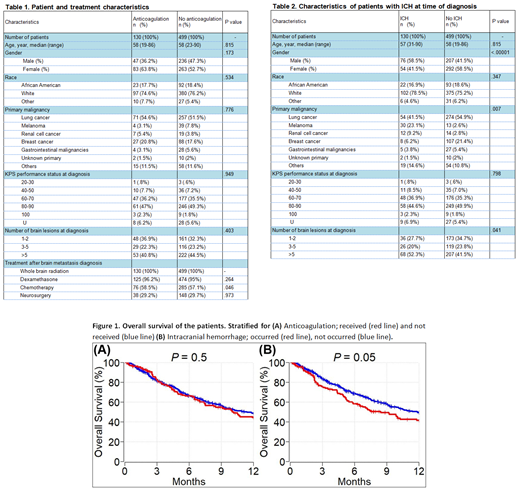
Results: We identified 903 cancer patients with brain metastasis. Of these, 629 (69.7%) met inclusion criteria. Among all included patients, 130 (20.7%) received therapeutic anticoagulation (AC) [enoxaparin: n = 75 (57.7%), warfarin: n = 41 (31.5%), apixaban/rivaroxaban/fondaparinux: n = 7 (5.4%), and unfractionated heparin: n = 7 (5.4%)] due to VTE after diagnosis of brain metastasis, 499 (79.3%) patients did not receive AC. Patient baseline and treatment characteristics for both cohorts are shown in Table 1. Deep vein thrombosis and pulmonary emboli were identified in 133 (21.1%) and 77 (12.2%) patients respectively.
ICH was recorded in 130 (20.7%) patients at the time of brain metastasis diagnosis. In the AC cohort, 22 of 130 (16.9%) patients had ICH, of which 13 (59.1%) clinically significant and 9 (40.9%) clinically insignificant at the time of brain metastasis diagnosis. In the no-AC cohort, 108 of 499 (21.6%) patients had ICH, of which 79 (73.1%) clinically insignificant and 29 (26.9%) clinically significant at the time of brain metastasis diagnosis. Median follow-up time of patients was 8.2 months. During the follow up time, ICH was detected in 14 (2.2%) patients overall, 12 (85.7%) patients were in the AC group and the other two (14.3) patients were in the no-AC group. Ten (71.4%) patients had clinically significant ICH.
In univariable analysis, male gender [p = 0.001] and primary cancer subtype (melanoma and renal cell cancer vs others) [p = <.001] were predictors of ICH at the time of brain metastasis diagnosis. In multivariable analysis, both gender and primary malignancy subtype [p <0.001 and .007 respectively] remained statistically significant predictors of ICH at the time of brain metastasis diagnosis (Table 2). In multivariate analysis, only number of brain lesions was a predictor of clinically significant ICH at the time of brain metastasis diagnosis [p=.036]. In multivariate analysis, ICH at diagnosis, therapeutic ACs use, type of ACs, and duration of ACs were significant predictors of ICH after brain metastasis diagnosis [all p <.0001]. None of the patients who had ICH received heparin, 42.9% received warfarin, 35.7% received enoxaparin, and 7.1% received direct oral anticoagulant (DOACs) [p <.0001].
One-year OS of patients with brain metastasis who received anticoagulation and did not receive anticoagulation was 44% (95% CI: 36 - 54) and 49% (95% CI: 44 -54), respectively (p=0.5) (Figure 1A). One year OS of patients with brain metastasis who had ICH and did not have ICH was 42% (95% CI: 33 - 52) and 49% (95% CI: 45 - 54), respectively (p=0.05) (Figure 1B).
Conclusion: In this cohort study, melanoma, renal cell cancer and male gender were significantly associated with increased risk of ICH. The use of therapeutic anticoagulation and history of ICH were associated with a greater risk of clinically significant ICH without affecting overall survival. About 88% of patients who had ICH during the follow up period received either warfarin or enoxparin. Larger studies are needed to better understand the safety profile of DOACs in this setting.
Khorana:Janssen: Consultancy; Bayer: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.