[Background] Glycosylphosphatidylinositol-anchored proteins (GPI-APs) on hematopoietic stem progenitor cells (HSPCs) may have some roles in the negative regulation of the HSPC commitment induced by inflammatory cytokines given the fact that progenies of GPI(-) HSPC are often detected in patients with immune-mediated bone marrow (BM) failure. CD109, one of the GPI-APs expressed by keratinocytes and HSPCs in humans, serves as a TGF-β co-receptor and is reported to inhibit TGF-β signaling in keratinocytes; however, the role of CD109 on HSPCs remains unknown. We previously demonstrated that TGF-β induced erythroid differentiation of TF-1 cells, a myeloid leukemia cell line that expresses CD109, in a dose-dependent manner and that knockout of the CD109 gene resulted in erythroid differentiation of TF-1 cells cultured in fetal bovine serum-containing medium, suggesting an inhibitory role of CD109 in the erythroid differentiation of HSPCs induced by low levels of TGF-β (Blood, 2018. 132 (Suppl.1) :3874). However, as most CD109 KO TF-1 cells changed into erythroid cells, they were unsuitable for investigating the role of CD109 in the erythroid differentiation induced by TGF-β. To overcome this issue, we prepared TF-1 cells and cord blood (CB) HSPCs in which the CD109 expression was transiently downregulated, and attempted to further clarify the role of CD109.
[Methods] TF-1 cells and CD34+ cells isolated from CB mononuclear cells were treated with siRNA that was complementary to CD109 mRNA. CD109 knockdown cells were cultured for 4 days in serum-free medium supplemented with stem cell factor, thrombopoietin, and erythropoietin with or without TGF-β. In separate experiments, TF-1 cells were treated with phosphatidylinositol-specific phospholipase C (PIPL-C) treatment for 1 hour and were incubated in the presence or absence of TGF-β. CD109 KO TF-1 cells were incubated in serum-free medium (StemPro-34 SFM) for 14 days and their phenotype was determined using flow cytometry (FCM). The erythroid differentiation of the cells was assessed by testing the expression of glycophorin A (GPA) and iron staining.
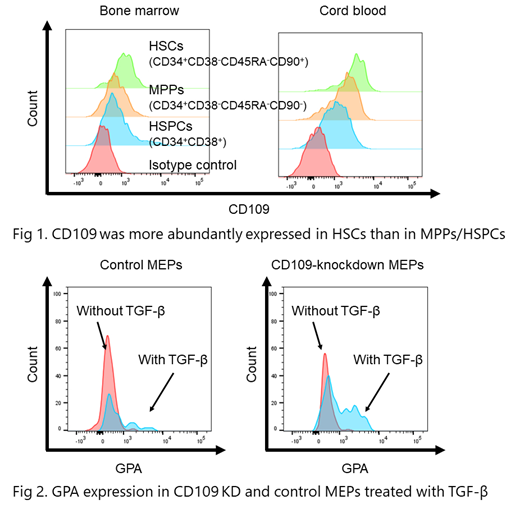
[Results] The down-regulation of CD109 in TF-1 cells by the siRNA treatment increased GPA expression in response to 12 ng/ml of TGF-β from 1.77% to 35.6%. The transient depletion of GPI-APs by PIPL-C also augmented the GPA expression induced by TGF-β from 1.27% to 6.77%. In both BM of healthy individuals and CB, CD109 was more abundantly expressed in Lin-CD34+CD38-CD90+CD45RA- hematopoietic stem cells (HSCs) than in Lin-CD34+CD38-CD90-CD45RA- multipotent progenitors (MPPs) and Lin-CD34+CD38+ HSPCs (Fig. 1). The treatment of CB cells with siRNA reduced the CD109 expression in Lin-CD34+CD38+ cells from 55.9% to 23.1%. TGF-β induced the expression of GPA in Lin-CD34+CD38+CD123-CD45RA- megakaryocyte-erythrocyte progenitor cells (MEPs) of CD109 knockdown cells to a greater degree than the control counterpart (Fig. 2). During 14-day serum-free culture, GPA-positive CD109 KO TF-1 cells died, and similarly to WT TF-1 cells, most surviving CD109 KO TF-1 cells were GPA-negative. TGF-β treatment induced erythroid differentiation in CD109 KO TF-1 cells to a greater degree than in WT TF-1 cells.
[Conclusions] CD109 plays a key role in the inhibition of TF-1 erythroid differentiation in response to TGF-β. CD109 may suppress TGF-β signaling, and the lack of CD109 may make PIGA-mutated HSPCs more sensitive to TGF-β, thus leading to the preferential commitment of the mutant erythroid progenitor cells to mature red blood cells in immune-mediated BM failure.
Yamazaki:Novartis Pharma K.K.: Honoraria; Sanofi K.K.: Honoraria; Nippon Shinyaku Co., Ltd.: Honoraria. Nakao:Novartis Pharma K.K: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceutical Company Limited: Honoraria; Celgene: Honoraria; Ono Pharmaceutical: Honoraria; Chugai Pharmaceutical Co.,Ltd: Honoraria; Kyowa Kirin: Honoraria; Alaxion Pharmaceuticals: Honoraria; Ohtsuka Pharmaceutical: Honoraria; Daiichi-Sankyo Company, Limited: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; SynBio Pharmaceuticals: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.