Introduction: Anemia affects over 2 billion people, which accounts for one-third of the world's population. Anemia causes numerous symptoms including weakness, fatigue, and dizziness as well as life-threatening cardiovascular collapse in severe cases. Primary hemoglobinopathies, including Sickle Cell Disease (SCD) and Thalassemia are the most common causes of anemia after iron deficiency and hookworm disease. Anemia induced by SCD and Thalassemia may potentially induce severe and chronic results thus both diseases require consistent monitoring. Optimal management of anemia and SCD specifically requires early diagnosis and consistent monitoring using blood tests that measure hemoglobin level and phenotype. Clinical hydroxyurea therapy in patients with SCD has been well established worldwide and an increase in hemoglobin level has been identified as an important clinical endpoint in assessing therapeutic efficacy. In a 2019 report, the World Health Organization (WHO) has listed hemoglobin testing as one of the most essential in vitro diagnostic (IVD) tests for primary care use in low and middle income countries. Furthermore, hemoglobin electrophoresis has recently been added to the WHO essential list of IVDs for diagnosing SCD and sickle cell trait. The current gold standard for anemia testing and hemoglobin level measurement is complete blood count (CBC) using a hematology analyzer. The current gold standard for hemoglobin variant identification is High Performance Liquid Chromatography (HPLC). Both tests require state-of-the-art laboratory infrastructure and highly trained personnel, which are typically scarce or non-existent in low- and middle-income countries, where anemia and hemoglobinopathies are most prevalent. As a result, there is a critical need for portable accurate affordable point-of-care tools for anemia detection and hemoglobin variant identification. Here, were present HemeChip+, a POC microchip electrophoresis technology for integrated anemia detection and hemoglobin variant identification in resource limited settings.
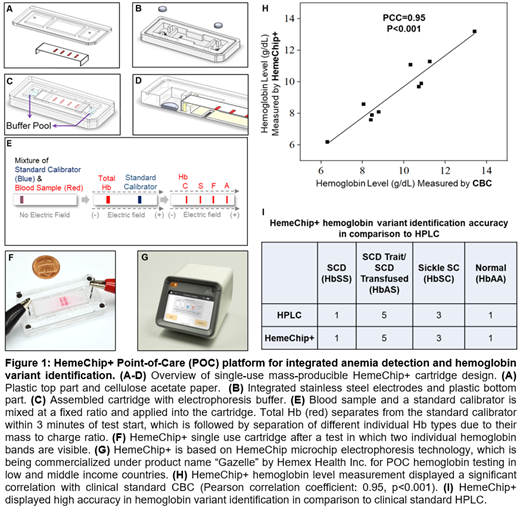
Methods: HemeChip+ works with a finger or heel prick blood sample. HemeChip+ test is embodied in a mass-producible fully integrated microchip electrophoresis cartridge (Fig. 1A-D). Integrated test is a two step process. Step 1 is the separation of the total hemoglobin (red) and the standard calibrator (blue), which takes place within 3 minutes of test start (Fig. 1E). This separation takes place due to the mass-to-charge ratio between hemoglobin and standard calibrator which were mixed at a fixed ratio. Total hemoglobin level is quantified by comparing hemoglobin band color intensity and the standard calibrator color intensity. Step 2 is the separation of hemoglobin variants (Fig. 1E), which takes place between 3 - 8 minutes after test start. In this step, individual hemoglobin sub-types separate according to their mass-to-charge ratio (Fig. 1E&1F). HemeChip+ platform has been developed based on extensively validated HemeChip microchip electrophoresis technology, which is currently being commercialized under product name "Gazelle" by Hemex Health Inc. for POC hemoglobin testing in low and middle income countries (Fig. 1G).
Results and Discussion: Feasibility of integrated total hemoglobin detection and hemoglobin variant identification was tested using blood samples from 10 donors with hemoglobin levels that ranged from 6.2 to 13.2 g/dL and various hemoglobin variants including normal (Hb AA), sickle trait (Hb AS), sickle cell disease (Hb SS), sickle hemoglobin C disease (Hb SC). HemeChip+ total hemoglobin measurement results were compared with clinical standard CBC tests. The results showed a Pearson correlation coefficient (PCC) of 0.95 (p<0.001) between HemeChip+ measurement and standard CBC (Fig. 1H). HemeChip+ hemoglobin variant identification results were compared with clinical standard HPLC results demonstrating 100% accuracy (N=10, Fig. 1I). HemeChip+ satisfactorily performed integrated anemia detection and hemoglobin variant identification in this study. HemeChip+ technology offers an original and innovative solution to integrated POC testing for anemia and hemoglobin disorders within the same platform.
An:Hemex Health, Inc: Consultancy, Equity Ownership, Patents & Royalties. Hasan:Hemex Health, Inc.: Equity Ownership, Patents & Royalties. Gurkan:Hemex Health, Inc.: Consultancy, Employment, Equity Ownership, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.