Background. Relapsed/refractory(R/R) AML has poor prognosis and lacks of standard treatment, while clinical trial has been recommended for these patients. It is necessary to find innovation and effective chemotherapy regimens for R/R AML. Cladribine-based regimens have been proved to be effective in R/R AML. As low-dose cytarabine (Ara-C) could reduce marrow myeloblasts and ATRA may sensitize leukemic cells to Ara-C, we performed the phase 1/2, single-arm, multicenter study of cladribine in combination with Ara-C,ATRA,G-CSF and mitoxantrone (CLAAG-M Regimen) in R/R AML (ChiCTR-OIC-16008110).
Methods. R/R AML patients aged 18-70 years were eligible to enroll in this study. The objective was to assess the responses and safety of this therapy. All patients received continuous infusion cladribine 5mg/m2 on days 1-5 or 1-3 and mitoxantrone 6mg/m2 on days 1,3,5 or 1,3. ATRA was taken orally at 45mg/m2 on day 4-6 and 15mg/m2 on days 7-20. Subcutaneous injection of cytarabine 15mg/m2 every 12 hours on days 1-10 and G-CSF 300ug/d on day 0 until disease remission or absolute neutrophils count ≥ 0.5×109/L were administered. All patents received one or two courses of induction treatments. Consolidation chemotherapy consisted of cladribine, low-dose Ara-C and G-CSF with or without mitoxatrone or ATRA according to the choice of study site.
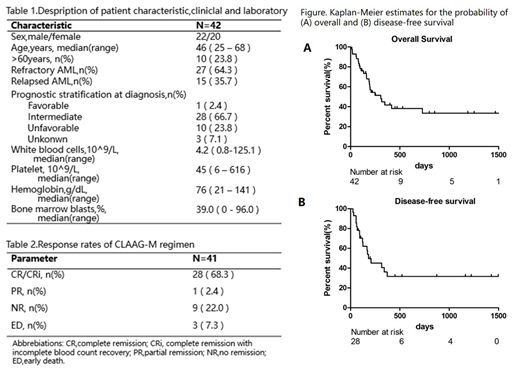
Results. Between April 2014 and July 2019, a total of 52 patients from five centers were screened,data of 7 patients who did not meet the enrollment criteria and 3 patients did not follow the established treatment were excluded. Therefore, 42 patients were enrolled in this study with 22 females and 20 males (Table 1). Median follow-up duration is 202.0 days(range 12.0-1560.0) from enrollment. The median age was 46 yr (range 25-68 ) . 27 out of 42 patients were refractory AML, with 15 patients in relapsed disease.One patient did not evaluate curative effect owing to personal reason,thus among 41 estimated patients, the overall response rate (ORR= CR+PR) was 70.7%, with complete remission rate (CRR = CR + CRi) of 68.3%, while early death rate and non-remission rate were 7.3% and 22.0% respectively (Table 2). For patients achieved CR, 10 of them (24.4%) received hematopoietic stem cell transplantation (HSCT) and more patients (36.6%) were only treated with consolidation therapy. The median overall survival (OS) of all patients was 306.0 days (95%CI 150.0-462.5) and the rate of 1-year and 2-year OS were 38% and 32% (Figure A). The median disease-free survival(DFS)was 183.0 days (95%CI 16.7-349.2) with half-year survival rate of 37% and 1-year survival rate of 32% (Figure B). Hematological toxicity was the most prominent toxicity of the CLAAG-M regimens since the median duration of neutrophils<0.5×109/L and platelets<20×109/L were 22 days (range8-42) and 17 days (range5-112),respectively. WHO>2 extra-hematological toxicities were infection (21/51) and bleeding (2/51). Three patients (7.3%) died early,with one from infection and two from organ hemorrhage. In multivariate analysis, transplantation would prolong the OS (P=0.013,95%CI of HR 0.010-0.578) .
Conclusion.CLAAG-M regimen is effective and tolerated in patients with R/R AML and could be as a bridging therapy regimen followed by HSCT. To confirm the clinical benefit, a randomized study will be further carried out.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.