Background: Apoptotic cell death can be triggered by activation of extrinsic and intrinsic signaling pathways. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, binds to its death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5) to selectively activate the extrinsic apoptotic pathway in cancer cells. ABBV-621 is a second-generation TRAIL receptor agonist with antitumor activity as monotherapy (621-mono) in preclinical models of AML and DLBCL. The intrinsic apoptotic pathway is regulated by the BCL2 protein family, commonly overexpressed in hematologic malignancies. Venetoclax (VEN), a highly selective small-molecule BCL2 inhibitor, has shown antitumor activity in combination therapy in AML and DLBCL. In preclinical and xenograft models of AML and DLBCL, the ABBV-621 and VEN combination (621-VEN) had antitumor activity superior to either agent alone. This first-in-human study evaluated ABBV-621 as single agent and in combination in pts with advanced solid tumors and hematologic malignancies (NCT03082209). Safety and tolerability of ABBV-621 in advanced solid tumors have previously been presented (Ratain et al. J Clin Oncol 2019;37[suppl]: abstr 3013). Here, we report preliminary data for 621-mono in pts with RR AML and for 621-VEN in pts with RR AML and DLBCL.
Methods: Adult pts with RR AML or DLBCL (ECOG 0-2) were enrolled. Pts with AML received ABBV-621 at 1.25-, 3.75-, 7.5-mg/kg doses in the 621-mono and at 3.75-mg/kg dose in the 621-VEN arms. Pts in the DLBCL 621-VEN arm received ABBV-621 at 3.75- and 7.5-mg/kg doses. ABBV-621 was administered intravenously on D1, 8, and 15 of a 21-D cycle; in 621-VEN cohorts pts received 400 mg oral VEN daily, and could be escalated to 800 mg. The primary endpoint was safety. In addition, preliminary antitumor efficacy and ABBV-621 binding to decoy receptors on neutrophils from peripheral blood were assessed.
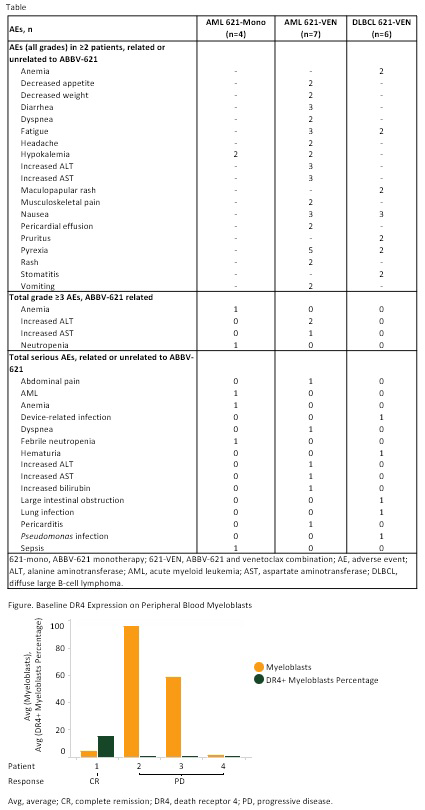
Results: As of Jun 2019, 17 pts were enrolled. Pts in AML 621-mono arm (1.25 [1], 3.75 [1], 7.5 [2] mg/kg): 1 male; median (med) age, 75 yr (range 71-82); med prior treatments, 2.5 (range 1-4); med time on treatment, 15 D (range 1-70). Pts in AML 621-VEN arm (3.75 mg/kg [7]): 5 male; med age, 71 yr (60-79); med prior treatments, 2 (1-2); med time on treatment, 26 D (1-77). Pts in DLBCL 621-VEN arm (3.75 [3], 7.5 [3] mg/kg): 4 male; med age, 57 yr (40-75); stage 4 (3); med prior treatments, 2.5 (1 to ≥5); med time on treatment, 26 D (8-36). One pt in the AML 621-VEN 3.75-mg/kg dose cohort had increases in alanine aminotransferase, aspartate aminotransferase and bilirubin as dose-limiting toxicities. Sixteen pts experienced adverse events (AEs) irrespective of causality. A summary of AEs is shown in Table. One pt in the AML 621-mono 7.5-mg/kg dose cohort died due to AML progression, unrelated to ABBV-621. Antitumor activity was observed in 1 pt in the AML 621-VEN arm (with complex cytogenetics and TP53 mutation) who reached complete remission (CR). One pt in the DLBCL 621-VEN 3.75-mg/kg cohort had stable disease, 2 with AML had resistant disease (1 in 621-mono [7.5 mg/kg] and 1 in 621-VEN), and 7 had progressive disease (PD; 1 in AML 621-mono [1.25 mg/kg], 2 in AML 621-VEN, and 4 in DLBCL 621-VEN [2 each in 3.75- and 7.5-mg/kg] cohorts). Using flow cytometry, saturation of ABBV-621 binding to decoy receptors on neutrophils was observed at 2 h postdosing, followed by dose-dependent desaturation of receptors in pts with DLBCL at 48-168 h. In AML 621-VEN pts, ABBV-621 remained bound to decoy receptors for up to 168 h; in DLBCL 621-VEN pts, the duration of binding was higher at ABBV-621 7.5 mg/kg compared with 3.75 mg/kg. In AML, the baseline frequency of myeloblasts was higher in pts with PD than CR, while that of myelomonocytes was higher in the pt with CR. The frequency of myeloblasts and myelomonocytes expressing DR4 and DR5 at baseline was highest in the pt with CR (Figure).
Conclusions: ABBV-621 was tolerated and showed antitumor activity in combination with VEN in pts with RR AML.
de Jonge:Faron Pharmaceuticals Ltd.: Consultancy. Carneiro:Actuate Therapeutics: Research Funding; Bayer: Research Funding; Pfizer: Research Funding; AstraZeneca: Research Funding; Medimmune: Research Funding; Astellas: Research Funding; AbbVie: Research Funding. Devriese:MSD: Consultancy. Penugonda:AbbVie: Employment, Other: Stock/stock options. Petrich:Abbvie: Employment, Equity Ownership. Nuthalapati:AbbVie: Employment, Other: Stock/stock options. Motwani:AbbVie: Employment, Other: Stock/stock options. Modi:AbbVie: Employment, Other: Stock/stock options. Chang:AbbVie: Employment, Other: Stock/stock options. Calvo:Celgene: Consultancy; Roche/Genentech: Consultancy, Other: travel/accommodations/expenses; Seattle Genetics: Consultancy; AstraZeneca: Consultancy, Research Funding; PsiOxus: Consultancy; Amcure: Consultancy; START: Other: stock/ownership interests, Research Funding; Janssen-Cilag: Consultancy; EUSA Pharma: Consultancy; Novartis: Consultancy, Research Funding, Speakers Bureau; Oncoart Associated: Other: stock/ownership interests; Guidepoint Global: Consultancy; Nanobiotix: Consultancy; HM Hospitales Group: Honoraria; AbbVie: Consultancy; BeiGene: Research Funding; Servier: Consultancy; International Cancer Consultants: Other: stock/ownership interests; Foundation INTHEOS: Other: president and founder; Gerson Lehrman Group: Consultancy; Pfizer: Consultancy. Moreno:Puma Biotechnology: Consultancy; Sanofi/Regeneron: Other: travel/accommodations/expenses.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract