Eighty-six newly diagnosed Philadelphia-negative ALL pts were enrolled from 2012 to 2018, from 14 Australian centres; 82 pts were evaluable. Pts were stratified and treated as per the pediatric ANZCHOG Study 8 protocol based on BFM 2000. Response was assessed on day 33 and 79 by morphology, flow cytometry and RQ-PCR measurable residual disease (MRD) at a central lab according to EuroMRD criteria. Allogenic stem cell transplantation was permitted for high and very high-risk disease groups. Detailed genomic analysis was performed in 47 pts (to date), using whole transcriptome sequencing (mRNA Seq) and multiplex ligation-dependent probe amplification (MLPA) for recurrent ALL related gene deletions.
Median age of the study was 24 (16 - 38) years; 28% were female; 59/82 (72%) had B-ALL. Median follow up was 36 (range 3-73) months. Induction mortality was 3.6%. CR rate at day 33 was 90.4% and day 79 (time point 2, TP2) 97.6%. Relapse free survival (RFS) at 2 years was 75.6% (95%CI 65.6 - 85.5%). CR rates at day 33 and day 79 were 90.4% and 97.6% respectively. The 2-year overall survival (OS) was 79.3% (18/82 events). In concordance with other studies, TP2 MRD predicted outcome in ALL06. MRD positive (pos) pts had a 2yr RFS of 68%, vs 98% in MRD negative (neg) pts (p=0.003).
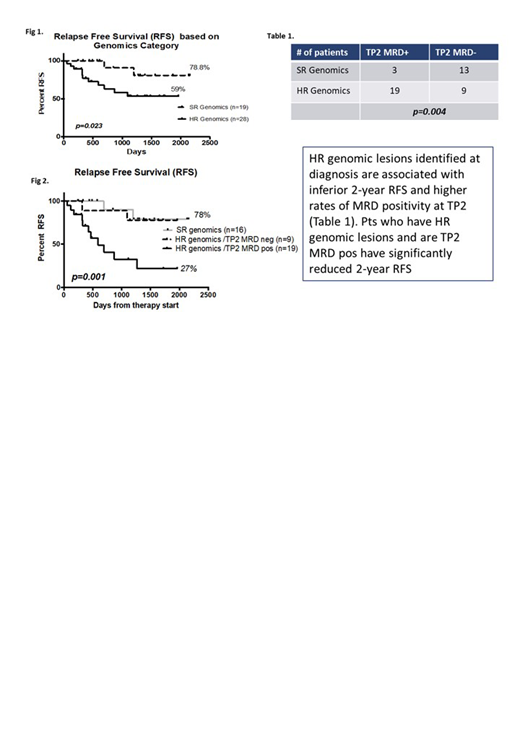
To date, 47 pts had mRNA Seq & MLPA; 11/47 pts had T cell ALL; 1/47 died during induction (2.1%). The median age of this subset was 21 (15-37) years, 23% were female and the RFS at 2 years was 73.97% (95%CI 65.6 - 91.44%). TP2 MRD remained predictive of outcome in this group with 2-year RFS in MRD pos pts 54% vs 95% in MRD neg pts (p=0.013, n=44). 13/47 pts have died with a 2-year OS of 73% (95%CI 62.7 - 90%). MPLA and mRNA Seq analysed independently of outcome data revealed 28/47 pts had genomic lesions categorise as High Risk (HR). These included fusions and structural genomic abnormalities involving KMT2A, IKZF1, IGH, ABL1, JAK, CRLF2, CDKN2A/B, PAX5, RAS and ZNF384. The remaining cases were classified as Standard Risk (SR) and included mainly hyperdiploid, T cell and ETV6-RUNX1 cases. Eleven of 13 pts who relapsed were genomic HR with poorer 2-RFS vs SR (59% vs 78.8%, p=0.023 respectively) (Fig 1.).
We examined the relationship between genomics risk group and TP2 MRD, a known prognostic marker. Of the 22 pts who were MRD pos, 19 (86%) pts were in the HR genomics group. In contrast, for MRD neg pts, 13/22 were in the SR group (59%) (p=0.004 Fishers exact, Table 1). This demonstrates that the TP2 MRD positive group is strongly enriched for pts with HR genomics. Pts with HR genomics who were TP2 MRD pos had a 2 year RFS of 27% vs HR MRD neg or SR pts with a 2 yr RFS of 78% (p=0.001)(Fig. 2). Further, of the 13 deaths that were observed in this subset 9/13 (69%) fell within the group of pts with HR genomics/TP2 MRD+. The single induction mortality, for whom TP2 data was not available was also genomic HR.
This is one of the first genomic surveys in a cohort of AYA pts, a group known for their inferior outcomes compared to children, treated on a pediatric inspired ALL protocol. Our overall outcomes compare favourably to other cohorts (EHA 2019 abstract 2416). In ALL06, genomic risk stratification based on previous published HR lesions, identified a HR cohort with significantly lower RFS and trend for inferior OS, vs a SR cohort. HR genomics was also associated with significantly higher rates of TP2 MRD positivity. Elucidation of targetable genomic lesions at the time of diagnosis may allow interventions to minimise MRD positivity and relapse in HR pts. Genomic information also improves understanding of underlying disease biology, providing targets for novel treatment discovery.
Yeung:Pfizer: Honoraria; Amgen: Honoraria; Novartis: Honoraria, Research Funding; BMS: Honoraria, Research Funding. Greenwood:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Wei:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: AHW is a former employee of the Walter and Eliza Hall Institute and receives a fraction of its royalty stream related to venetoclax, Research Funding, Speakers Bureau; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Macrogenics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Research Funding; Janssen: Honoraria. White:AMGEN: Honoraria, Speakers Bureau; BMS: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.