Introduction
Extranodal NK/T cell lymphoma (NKTCL) is an aggressive malignance that is correlated closely with persistent Epstein-Barr virus (EBV) infection and belongs to a latency II EBV disease. Chronic persistent viral infection is known to result in T cell exhaustion, which will dramatically impede anti-tumor immunity. Delineating mechanisms of immunosuppression contributes to the development of novel immuno-therapeutically strategies. Herein, we described the immune status and possible role of latent membrane protein 1(LMP1) in NKTCL.
Methods
Peripheral blood mononuclear cells (PBMCs) from newly diagnosed NKTCL patients and age-matched healthy donors (HDs) were isolated and stained with surface marker or intracellular marker after permeabilization. The frequency of EBV-specific cytotoxic T cells (CTLs) was analyzed by staining with HLA-A2 tetramers assembled with synthetic peptides from LMP1. T cell proliferation was detected by dilution of stained CFSE, and apoptosis of T cells was performed via annexinV and 7-AAD dual-staining. Cytotoxicity assay of natural killer cells (NK) was measured by detecting apoptosis of CFSE-labeled K562 cells after co-culturing with PBMCs. All flow cytometry was performed by BD FACS CantoII, and analyzed with FlowJo software.
Results
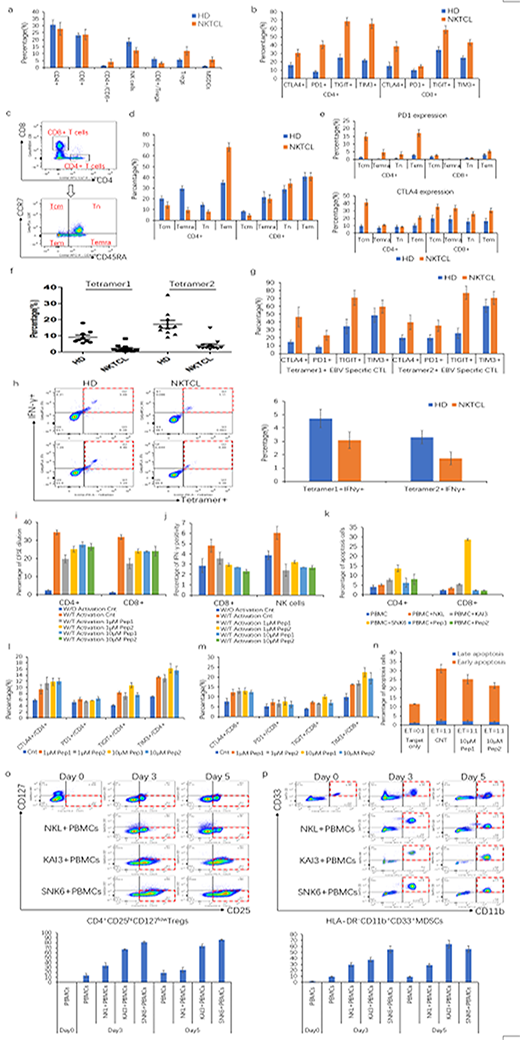
PBMCs from 19 NKTCL patients and 18 HDs were analyzed, which showed that the patients had higher ratio of CD4+/CD8+ cells and lower frequency of CD3-CD56+NK cells, higher percentage of immunosuppressive CD4+CD25hiCD127low T regulatory cells (Tregs) and HLA-DR-CD11b+CD33+ myeloid derived suppressor cells (MDSCs). Notably, the ratio of CD8+/Tregs, a parameter representing immune effector cells status, was obviously decreased in NKTCL patients (Figure-a). Given the potential association of T cell exhaustion and NKTCL, we detected expression of T cell exhaustion markers on T cells from NKTCL patients, and found that both CD4+ and CD8+ T cells expressed much higher level of inhibitory molecules PD1, CTLA4, TIGIT and TIM3 in NKTCL patients than that of HDs (Figure-b). To further explore cellular immunity in NKTCL, T cells were divided into four subsets: CD45RA-CCR7+ central memory T cells (Tcm), CD45RA+CCR7+ terminally differentiated effector T cells (Temra), CD45RA+CCR7- naïve T cells (Tn), CD45RA-CCR7- effector memory T cells (Tem) (Figure-c). It showed that CD4+ T cells had lower Tcm, Temra and Tn but much higher proportion of Tem compared with HDs (Figure-d). Moreover, we found that expression of PD1 and CTLA4 were upregulated on all lymphocyte subsets in NKTCL patients than HDs (Figure-e).
We collected additional 13 NKTCL patients and 10 HDs with positive HLA-A2 phenotype to measure percentages of EBV-specific CTLs, which showed that frequency of EBV-specific CTLs was remarkably decreased in NKTCL patients (Figure-f). In addition, EBV-specific CTLs from NKTCL patients were more likely to express higher levels of exhaustion markers but produced much less IFN-γ (Figure g-h).
To explore the potent mechanism of immunosuppression in NKTCL, LMP1 attracted our attention, which had been proven capable of activating multiple signaling pathways to exert its oncogenesis function. To elucidate effect of LMP1 on immune cells, we constructed two LMP1-derived peptides and found that LMP1-derived peptides suppressed the proliferation of CD4+ and CD8+ T cells and inhibited IFN-γ production of CD8+ T cells and NK cells (Figure i-j). Meanwhile, LMP1 promoted apoptosis of both CD4+ and CD8+ T cells (Figure-k). Subsequently regulation of LMP1 on exhaustion markers of T cells were detected, which showed that PD1, CTLA4, TIGIT and TIM3 were significantly upregulated on both CD4+ and CD8+ T cells after treatment with LMP1 derived peptides (Figure-m). Furthermore, LMP1 impaired NK cytotoxicity ability, evidenced by decrease apoptosis of target cells (Figure-n).
Besides its effect on immune effector cells, we also found that LMP1-expressing NKTCL cell lines obviously induced both Tregs and MDSCs after co-culture. Interestingly, the extent of induction by LMP1 of Tregs and MDSCs were in line with the expression level of LMP1 on NKTCL cells (Figure o-p).
Conclusion
Our study showed that LMP1 contributes to deficient cellular immunity in NKTCL patients, providing us with more insight into immunosuppressive in this entity of disease, which would lead to identification of novel treatment strategies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.