Introduction
Approximately 20% of patients (pts) with classical Hodgkin lymphoma (cHL) are age ≥ 60 years (yrs) at diagnosis (dx). Standard anthracycline-based therapies (ABTx) have historically shown lower efficacy and greater toxicity in this older population compared with younger pts, especially for unfit or frail pts. However, prior analyses have pre-dated the use of brentuximab vedotin (BV) and other novel targeted agents in the front-line setting and there remains no clearly defined or unified standard of care for this pt population. Studies in older lymphoma pts have also defined specific categories of pt fitness that may potentially guide therapy, although continued validation of these definitions in cHL are needed. We performed a large, multicenter retrospective study to identify current practice patterns in the US in older untreated cHL pts with specific attention to pt fitness using established definitions and to outcomes with ABTx versus non-ABTx.
Methods
Following IRB approval at each site, detailed pathologic and clinical data, including geriatric assessments (GAs) for the latter, were collected across 10 US academic medical centers. Eligible pts included: cHL dx on or after 1/1/2010, age ≥ 60 yrs at dx, and no preceding hematologic malignancy. Subjects with inadequate clinicopathologic and outcomes data or with dx other than cHL were excluded. A multivariable Cox proportional hazards model was developed to study the association of PFS and OS with various factors of interest, and survival rates were estimated by Kaplan-Meier using SPSS. Associations were considered significant for two-sided P values ≤ 0.05.
Results
Among 254 eligible older cHL pts with newly diagnosed disease, clinical features included: median age 67 yrs (range 60-90+), 54% male, 64% stage III/IV, 46% B symptoms, and 86.1% ECOG PS 0-1. 68.9% had nodular sclerosis, 18.9% mixed cellularity, and 12.2% other subtypes; EBV was detectable by IHC or peripheral blood in 39.3% of evaluable cases (n=98).
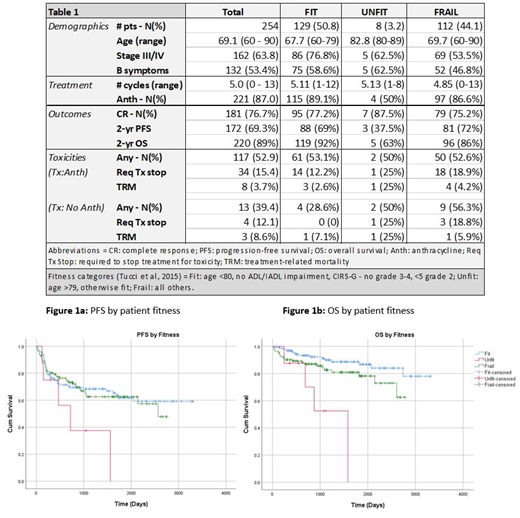
In terms of geriatric measures, 31 pts (12.2%) had loss of at least one ADL; 40 (15.8%) had a geriatric syndrome; and 16 (6.3%) had both. Of note, only one pt underwent a comprehensive GA prior to treatment. Using established fitness definitions in lymphoma (Tucci et al, 2015), pts were classified as "Fit" (n=129; 50.8%), "Unfit" (n=8; 3.2%), or "Frail" (n=112 (44.1%); 5 (2%) were unclassifiable due to incomplete data (Table 1).
Front-line regimens included ABVD (n=145; 57.1%), AVD (n=33; 13.0%), and CHOP (n=18; 7.1%). 6 pts (2.4%) received BV monotherapy; 21 (8.3%) received BV with AVD, the latter either concurrently (N=11) or sequentially (n=10). 11 pts did not receive systemic tx, of whom 9 received radiation alone; 58 pts received radiation with systemic tx. ABVD recipients received an average of 7.4 doses of bleomycin (range 1-14) and 18.4% (n=26) had pulmonary toxicity. In addition, 56.7% (n=130) of pts experienced at least one tx-related toxicity, which was similar across all fitness groups (Table 1). 15.1% (n=38) of pts stopped tx due to toxicity, which was noted more often in "frail" pts though it was relatively uncommon. 11 pts (4.3%) experienced treatment-related mortality (TRM).
Compared with pts receiving non-ABTx, ABTx was associated with significantly improved PFS (HR 0.391, P=0.008) and OS (HR 0.195, P=0.0004). ECOG PS >1 was associated with inferior PFS (HR=1.96, P=0.017) and OS (HR=3.09, P=0.002). Pts defined as "frail" had no apparent difference in PFS compared with fit pts (Figure 1A;P=0.169), although decreased OS was seen (Figure 1B; P=0.01).
Discussion
To the best of our knowledge, these data represent one of the largest analyses of older cHL pts in the modern era. Our real-world analysis shows that in those who are able to receive them, anthracyclines provide meaningful survival benefit in this older cHL patient population and can be well-tolerated for many pts. The impact of dose intensity and backbone regimen are not yet known. In addition, current definitions of "fitness" incompletely identified pts at risk for poor outcomes or greater toxicity. Further studies are planned to better delineate fitness in this population.
Bartlett:Celgene: Research Funding; ADC Therapeutics: Consultancy, Research Funding; Affimed: Research Funding; Merck: Research Funding; Janssen: Research Funding; Immune Design: Research Funding; Gilead: Research Funding; Millenium: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; Forty Seven: Research Funding; Genenetech: Research Funding; Bristol-Myers Squibb: Research Funding. Grover:Seattle Genetics: Consultancy. Bennani:Adicet Bio: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Adicet Bio: Other: Advisory board; Seattle Genetics: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board. Hill:Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria; Celegene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Takeda: Research Funding; Amgen: Research Funding; TG therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Research Funding. Advani:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc./Kite Pharma, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Millennium: Research Funding; Kura: Research Funding; Janssen: Research Funding; Autolus: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Regeneron: Research Funding; Infinity Pharma: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Celmed: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Kirin Pharmaceutical Developments, Inc.: Consultancy; Agensys: Research Funding; Stanford University: Employment, Equity Ownership; Seattle Genetics: Consultancy, Research Funding; Cell Medica, Ltd: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Svoboda:AstraZeneca: Consultancy; Celgene: Research Funding; Incyte: Research Funding; Pharmacyclics: Consultancy, Research Funding; Kyowa: Consultancy; Merck: Research Funding; BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding. Magarelli:Tevan Oncology: Speakers Bureau. Feldman:Takeda: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Travel expenses, Speakers Bureau; AbbVie: Honoraria, Other: Travel expenses, Speakers Bureau; Pharmacyclics: Honoraria, Other: Travel expenses, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Kite Pharma: Honoraria, Other: Travel expenses, Speakers Bureau; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Cell Medica: Research Funding; Roche: Research Funding; Corvus: Research Funding; Eisai: Research Funding; Kyowa Hakko Kirin: Research Funding; Pfizer: Research Funding; Portola Pharma: Research Funding; Roche: Research Funding; Trillium: Research Funding; Viracta: Research Funding. Cohen:Astra Zeneca: Research Funding; ASH: Research Funding; UNUM: Research Funding; Hutchison: Research Funding; Takeda Pharmaceuticals North America, Inc.: Research Funding; Gilead/Kite: Consultancy; Lymphoma Research Foundation: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics, Inc.: Consultancy, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Janssen Pharmaceuticals: Consultancy; Genentech, Inc.: Consultancy, Research Funding. Evens:Seattle Genetics: Consultancy, Honoraria, Research Funding; Tesaro: Research Funding; Epizyme: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria. Portell:AbbVie: Research Funding; Pharmacyclics: Consultancy; Janssen: Consultancy; Genentech: Consultancy, Research Funding; Amgen: Consultancy; Bayer: Consultancy; BeiGene: Consultancy, Research Funding; Kite: Consultancy, Research Funding; Acerta/AstraZeneca: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Roche/Genentech: Research Funding; Infinity: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.