Introduction
The addition of Ibrutinib to R-CHOP in younger pts with DLBCL has efficacy, but significant toxicity limits the ability to complete therapy in pts ≥60 yrs (Younes et al, JCO 2019). We have previously demonstrated the deliverability of Ibrutinib with R-mini-CHOP in 80 pts ≥75yrs with DLBCL with a median Average Relative Total Dose of 97%; 77% of pts received 6 cycles of R-mini-CHOP despite SAEs in 62% (Verner et al, Haematol Oncol 2019). Here we present response rates, Cumulative Illness Rating Scale-Geriatrics (CIRS-G) score, left ventricular ejection fraction (LVEF) and quality of life (QoL) data.
Methods
Pts received six 21 day cycles of ibrutinib 560mg/d and R-mini-CHOP (Rituximab 375mg/m2, cyclophosphamide 400mg/m2, doxorubicin 25mg/m2, vincristine 1mg on day 1 &prednisone 100mg/d x 5) followed by an additional two 21 day cycles of rituximab + ibrutinib (or high dose methotrexate for CNS prophylaxis). Baseline comorbidities assessed by CIRS-G, end of treatment (EOT) response assessment per Lugano 2014 criteria and QoL (EORTC-QLQ-C30 and EQ-5D-5L visual analogue scores (VAS)) are presented for screening, throughout therapy and 1-month post treatment, with differences assessed using ANOVA. Missing values were not imputed.
Results
80 pts were recruited from Nov 2015 to Dec 2018. Median age at registration was 81yrs (75-95); 51% female, 81% stage III/IV and 63% IPI 3-5. The majority of pts (88%; 70/80) had a CIRS-G score of ≥6 (range 4-21), a cut-off shown to be associated with a higher risk of early chemotherapy discontinuation in haematological malignancies (Poisson et al, EHA 2016). Median follow-up was 13.1 months (range 0.2 to 42.6). Treatment discontinuation occurred in 31% (25/80) pts. 23/80 (29%) have died; 14 due to progressive lymphoma, 5 due to infection, 1 intra-abdominal haemorrhage, 1 respiratory failure, 1 cardiac arrest, and 1 MPN/MDS. There was no association between baseline CIRS-G score, and completion of treatment, death or disease response.
The overall response rate (ORR) on an intention to treat basis was 57/80 (71%). Eight cycles of treatment were completed by 55/80 (69%) pts, of whom 54 had an EOT PET. Of this population there was an ORR of 93% (50/54), with complete metabolic response (CMR) in 47/54 (87%); partial metabolic response in 4/54 (7%); and progressive metabolic disease in 3/54 (6%). A further 6 pts who discontinued treatment early achieved a CMR (between cycles 3-6) and 1 had stable metabolic disease. Progressive disease prior to cycle 8 occurred in 7/80 pts (9%). Eleven pts had no treatment response assessment, either due to death (n=5), withdrawal of consent (n=3), loss to follow up (n=2), or death prior to commencing chemotherapy.
2/80 (2%) pts had a recorded ≥10% decline in LVEF to <50%. There was no significant difference in LVEF from baseline [mean 65.0% (SD 7.1)] to 3 months post EOT [mean 62.2% (SD 9.9)] p=0.21, in the 44 pts with both assessments available for comparison.
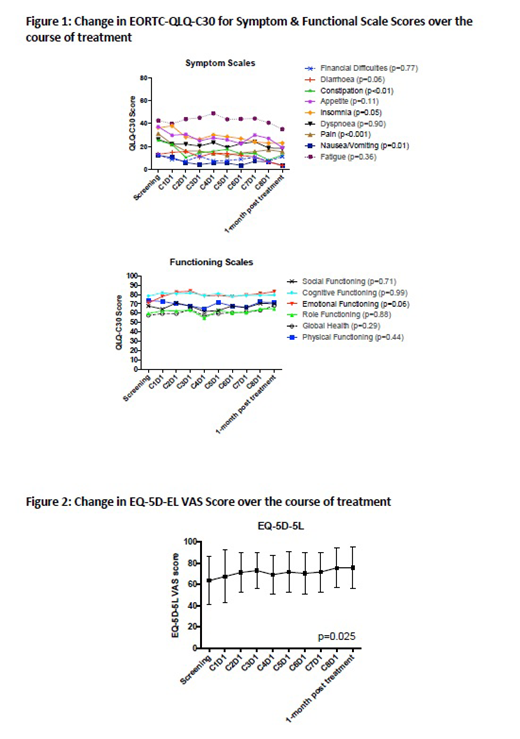
QoL outcomes are presented in Figures 1 and 2. QOL, as assessed by two globally accepted tools, improved with treatment in the population completing EOT assessment. Patients' EORTC-QLQ-C30 global health status and emotional functioning improved substantially while maintaining physical and cognitive functioning. There was a significant reduction in nausea, vomiting, pain, insomnia, constipation, and diarrhoea and an improvement in appetite with treatment. Similarly EQ-5D-5L VAS demonstrated that patients maintained mobility and independence with a significant reduction in pain/discomfort and improvement in mood.
Conclusions
Albeit with considerable toxicity and early mortality, Ibrutinib-R mini-CHOP is a deliverable and effective treatment for most pts ≥75yrs with DLBCL. While longer follow-up is required to assess our primary efficacy endpoint of 2yr overall survival, this promising response and reassuring QoL data highlights the merits of ongoing study to identify deliverable and effective treatments for DLBCL in the very elderly.
Verner:Janssen-Cilag Pty Ltd: Research Funding. Hawkes:Astra Zeneca: Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck Sharpe & Dohme: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding, Speakers Bureau; Merck KgA: Research Funding; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Speakers Bureau; Mundi pharma: Research Funding; Bristol-Myers Squibb: Research Funding, Speakers Bureau. Lee:Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria; MSD: Membership on an entity's Board of Directors or advisory committees. Cheah:F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; AbbVie: Research Funding; Celgene: Research Funding; Gilead: Honoraria; Janssen: Honoraria; Acerta: Honoraria; Loxo: Honoraria. Purtill:MSD: Honoraria; Novartis: Honoraria, Other: Travel for speaking and advisory boards; Gilead: Honoraria, Other: Travel for speaking and advisory boards; Janssen: Honoraria. Enjeti:Astellas: Consultancy; Novartis: Consultancy; Abbvie: Consultancy; Bayer and Sanofi: Honoraria, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Curnow:NovoNordisk: Honoraria; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CSL: Honoraria; Mylan Pharmaceuticals: Consultancy; Freeline: Membership on an entity's Board of Directors or advisory committees; Boehringher Ingelheim: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria; Pfizer/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Butcher:ALLG: Honoraria; Abbvie: Honoraria; Astellas: Honoraria; BMS: Honoraria; Janssen-Cilag Pty Ltd: Honoraria; MSD: Honoraria; Novartis: Honoraria; Roche: Honoraria; Shire: Honoraria; Sandoz: Honoraria. Trotman:Pharmacyclics: Research Funding; Celgene: Research Funding; Janssen: Research Funding; BeiGene: Research Funding; Roche: Research Funding.
Ibrutinib is not currently approved for use in DLBCL
Author notes
Asterisk with author names denotes non-ASH members.