Introduction: Non-endemic Burkitt lymphoma (BL) is a rare and highly aggressive B-cell malignancy, of whom a substantial number are adolescents and young adults (AYAs). In this particular group of patients, the balance between efficacy and long-term toxicities is of major concern, due to the long expected remaining lifetime. In this study, we investigated the outcomes for AYA patients treated with intensive immunochemotherapy.
Patients and methods: Patients were identified through queries to clinic based and population-based lymphoma registries from six countries (Australia, Canada, Denmark, Norway, Sweden, and USA). All diagnoses were confirmed by local investigators; patients with classical BL histology plus detectable MYC translocation, were included. Patients between 18 and 39 years of age at diagnosis treated with intensive immunochemotherapy (DA-EPOCH-R or more intensively) were evaluated in pre-specified age groups. All treatment protocols included rituximab. Overall survival (OS) was defined as the time from diagnosis until death from any cause or censoring, while event-free survival (EFS) was defined as the time from diagnosis until unplanned re-treatment, progression/relapse, death, or censoring, whichever came first. Response evaluation was assessed using established response criteria based on CT and/or PET/CT imaging. Survival curves were computed using the Kaplan-Meier estimator. The cumulative incidence of relapse in patients reaching complete remission (CR) or CR unconfirmed (CRu) was computed by Aalen-Johansen estimator. The 5-year restricted loss of lifetime (RLOL) was defined as the area between the patient and general population survival curves until 5-years. The general population survival was retrieved from publicly available lifetables stratified on age, sex, calendar year, and country.
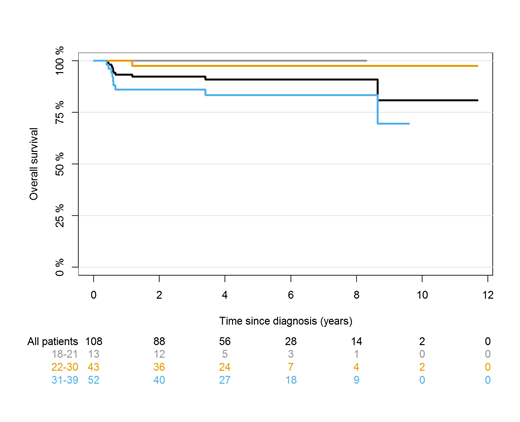
Results: In total, 108 AYA BL patients were included. The median age was 30 years, ranging between 18 and 39 and 82% were male. The majority had advanced stage disease (Ann Arbor stage III-IV, 76%), extranodal involvement (87%), and elevated LDH (67%). Seven patients (6%) had CNS involvement at the time of diagnosis. The chemotherapy regimens used were CODOX-M/IVAC (51%), BFM/GMALL (34%), hyper-CVAD (7%), DA-EPOCH (5%), and others (3%). Among 74 patients for whom data on tumor lysis were available, 10 (14%) developed clinical tumor lysis upon start of chemotherapy, all presenting with advanced stage and extranodal disease. The response rate was 91% (89 in CR/CRu and 2% in partial remission). At a median follow-up of 53 months, 15 had an event and 10 died. The 2-year OS and EFS for the total population was 92% (87-97%, Figure 1) and 88% (82-94%), respectively. For 13 patients aged 18-21 2-year OS and EFS were both 100%, while 2-year OS and EFS for 43 patients aged 22-30 were 98% (93-100%) and 93% (85-100%), respectively. The 2-year OS and EFS for patients aged 31-39 were 86% (76-96%) and 80% (69-91%), respectively. For patients in CR/CRu and available date of response evaluation, the 2-year post-remission OS was 99% (97-100%) and the 2-year relapse risk was 2% (0-5%), with no relapses after 6 months. Compared to the general population, the RLOL was 0.8 months (-0.5-2.2 months). Nine patients did not respond to primary treatment (SD or PD), all which belonged to the age group 31-40 years (P<0.001 versus age group 18-30 years). The oldest age group had an inferior OS compared to younger patients (P=0.008), largely due to non-responders having poor subsequent survival with a 1-year OS of 33%.
Conclusion: The outcomes of AYA BL patients following intensive immunochemotherapy are excellent with high remission rates and low risk of relapse in general. However, even among young patients age remained prognostic, with patients 31 years or older having inferior OS and lower response rate to first-line treatment. In patients achieving CR, survival was similar to that of the general population over the median follow-up of 4.5 years, illustrating the need to focus on survivorship issues in these individuals.
Figure 1: Overall survival for BL patients following intensive immunochemotherapy for all patients and for age groups (18-21, 22-30, and 31-39 years).
Jørgensen:Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Holte:Novartis: Honoraria, Other: Advisory board. Molin:Roche Holding AG: Honoraria; Merck & Co., Inc.: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceuticals: Honoraria. Ekstroem Smedby:Takeda: Honoraria, Other: Grant funding, Research Funding; Celgene: Honoraria, Other: Grant funding, Research Funding; Janssen Cilag: Honoraria, Other: Grant funding, Research Funding. Song:Takeda: Honoraria; Celgene: Honoraria, Research Funding; Janssen: Honoraria; Amgen: Honoraria. Gerrie:Lundbeck, Seattle Genetics: Consultancy, Honoraria. Cheah:Roche, Janssen, MSD, Gilead, Loxo Oncology, Acerta, BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Other: Travel expenses; Celgene, Roche, Abbvie: Research Funding. El-Galaly:Roche: Employment, Other: Travel support; Takeda: Other: Travel support.
Author notes
Asterisk with author names denotes non-ASH members.