Background: Successful tyrosine-kinase inhibitors (TKIs) discontinuation has been obtained in some patients (pts) with chronic-phase chronic myeloid leukemia (CP-CML). Careful molecular monitoring after discontinuation is the key to guarantee the safety, in terms of prompt resumption of therapy according to retreatment threshold criteria. It was observed that the majority of relapses usually occur during the first 6 months after TKI discontinuation [Saussele S, Lancet Oncol 2018; Etienne G, JCO 2017], accounting for the monthly quantitative PCR (qPCR) that all prospective protocols included in the trial design at least during the first half-year. Two studies [Kong HJ, Cancer 2017; Shanmuganathan N, Blood 2019] investigated if performing molecular analysis with a different and less "cautious" timeframe yields comparable efficacy with logistical issues reduction. Here we retrospectively evaluated how molecular monitoring has been conducted in Italy on a cohort of patients not included in any prospective trial with follow-up visits.
Methods: The outcome of Italian patients with CP-CML who discontinued TKIs per clinical practice has recently been reported [Fava C, Haematologica 2019]. For the purpose of the present study, all the 32 participating centers were required to provide dates and molecular results available for each enrolled patient in the first 24 months after TKI stop. Descriptive analysis was carried out. The average time to the loss of major molecular response (MMR), the frequency of the visits (monitoring) and the occurrence of loss of MMR within the first 6 months, between 6-12 months, and 13-24 months were computed. When appropriate non-parametric tests were used to test for differences.
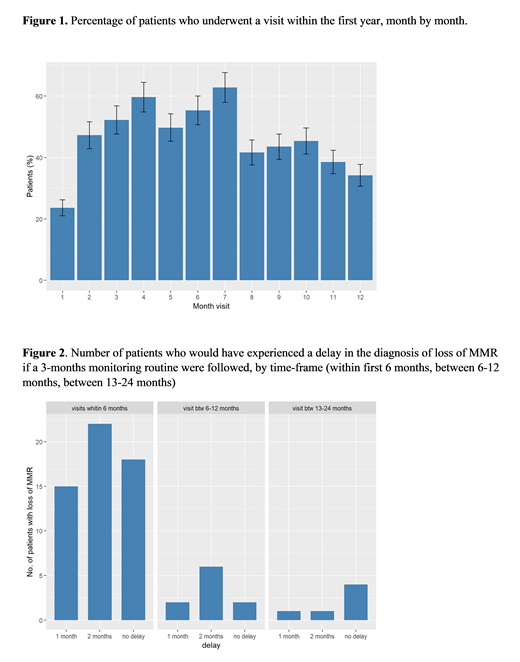
Results: 227 chronic phase CML pts were included in this sub-analysis. Median age at TKI discontinuation was 58.73 years and median follow up since TFR was 2.03 years. In this timeframe every patient had a mean of 7.95 appointments for molecular evaluation. Overall, 1804 analysis were performed, of which 18.2% happened in the first three months and 38.2% in the first six months. During the first three months of TKI discontinuation, 40 pts (17.6%) didn't have any molecular assessment; 78 pts (34.4%) had only 1 qPCR performed, 77 pts (33.9%) 2 qPCR, 31 pts (13.7%) 3 qPCR and 1 pt (0.4%) 4 qPCR. For the first six months after TKI stop, 7 pts (3.1%) didn't undergo any BCR-ABL1 evaluation; 37 pts (16.3%) had only 1 analysis, 60 pts (26.4%) 2 analysis, 37 pts (16.3%) 3 analysis, 28 pts (12.3%) were evaluated 4 times, 40 pts (17.6%) 5 times, 17 pts (7.5%) 6 times and only 1 pt (0.4%) 7 times. The majority of visits fell between the 3rd and the 7th month after TKI interruption (Figure 1) with 84 pts (52.2%) being evaluated at month 3, 96 pts (59.6%) at month 4, 80 pts (49.7%) at month 5, 89 pts (55.3%) at month 6, 101 pts (62.7%) at month 7. In the first six months the visits occurred with a mean interval of 1.44 months; between months 7-12 molecular evaluations were performed every 1.94 months; during the second year of discontinuation (months 13-24) every 2.89 months (p<0.001). Seventy-one pts lost major molecular response (MMR) in a mean time of 5.56 months. As expected, 55 pts (77.5%) lost MMR during the first six months whereas 16 pts (22.6%) relapsed later on: 3 pts (4.2) relapsed during the first month, 7 pts (9.9%) after two months, 13 pts (18.3%) after three, 19 pts (26.8%) after four, 8 pts (11.3%) after five months and 5 pts (7%) at six months. Only 6 patients lost MMR after 12 months of follow-up in TFR. All patients regained at least MMR after TKI resumption, and no progression occurred. Finally, we evaluated the number of patients who would experience a delay in the diagnosis of MMR loss if a three-months monitoring schedule was adopted. In the first 6 months, 15 pts (27.3%) would have a one month delay, 22 (40%) a 2 months delay; 18 pts (32.7%) would have a right timing. Very few patients would experience a delay in the following months (Figure 2).
Discussion: The safety of TFR relies consistently on the management of patients off-therapy especially during the first 6 months, when molecular relapses more often occur. Our retrospective analysis showed that a less intense frequency of monitoring did not affect the success of TFR nor put pts at risk of progression. However, these data confirm that the first 6 months off-treatment require a more stringent follow-up for early detection of MMR loss.
Rosti:BMS: Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Castagnetti:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Bristol Myers Squiib: Consultancy, Honoraria. Capodanno:Novartis: Honoraria; Incyte: Honoraria. Ferrero:Novartis: Honoraria. Crugnola:Novartis: Honoraria; Incyte: Honoraria. Elena:Pfizer: Consultancy; Novartis: Consultancy. Breccia:Pfizer: Honoraria; Celgene: Honoraria; Novartis: Honoraria; BMS: Honoraria; Incyte: Honoraria. Iurlo:Novartis: Other: Speaker Honoraria; Pfizer: Other: Speaker Honoraria; Incyte: Other: Speaker Honoraria. Bocchia:BMS: Honoraria; Incyte: Honoraria; Novartis: Honoraria. Lunghi:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Cedrone:BMS: Honoraria; Novartis: Honoraria. Sgherza:Pfizer: Honoraria; Novartis: Honoraria; BMS: Honoraria; Incyte: Honoraria. Santoro:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Giai:Pfizer: Honoraria; Novartis: Honoraria; BMS: Honoraria. Caocci:Novartis: Honoraria; Celgene: Honoraria. Levato:Incyte: Honoraria; BMS: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Abruzzese:BMS: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; Novartis: Consultancy. Saglio:Pfizer: Consultancy; Celgene: Consultancy; Incyte: Consultancy; Jansen: Consultancy; Ariad: Consultancy; Novartis: Consultancy; BMS: Consultancy. Fava:Pfizer: Honoraria; Novartis: Honoraria; BMS: Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.