Introduction:Generic imatinib formulations are increasingly being used as more affordable alternatives worldwide and a few studies have evaluated the safety and efficacy of these formulations prospectively. We have retrospectively analyzed our CML cohort in terms of first line treatment of Glivec versus generic imatinib. This study aims to evaluate the safety and efficacy of generic imatinib products in chronic phase chronic myeloid leukemia as first line treatment.
Methods:We have retrospectively analyzed our CML cohort from January 2000 to December 2018 treated with either Glivec or one of generic imatinib formulations. All of our patients (with 1 exception) were initiated imatinib in chronic phase in less than 56 days from diagnosis. All of our patients were followed in accordance with European Leukemia Net (ELN) 2013 recommendations and national hematology association CML guidelines and response definitions were applied according to ELN 2013 criteria. Event free survival (EFS) was defined as the time between treatment initiation and either loss of hematological response, progression to accelerated phase (AP) or blastic phase (BP), or death from any cause. Progression free survival was defined as the time between treatment initiation and transformation to AP, BP or death while on imatinib. For statistical analyses SPSS version 21.0 was used. All p values < 0.05 were considered statistically significant.
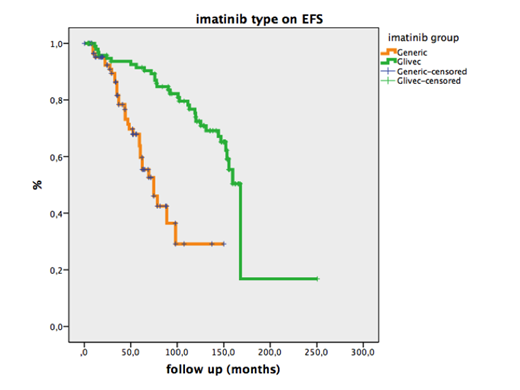
Results:A total of 192 patients were analyzed comparing 102 (53.1 %) patients on Glivec with 90 patients on (476.9 %) generic formulations. 99 (51.6 %) were female patients. The median age of our population was median 46 years (14-88 years) for Glivec and median 51 years (19-79 years) for generic group (p=0.01). Risk stratifications according to Sokal, Hasford and ELTS scores were run for both Glivec and generic formulation groups. Most of the patients had low risk according to Sokal (137, 71.4%) and Hasford (116, 60.4 %) but intermediate risk according to ELTS (113, 58.9 %) scoring systems. There was no statistically significant difference in the gender distribution, Sokal, Hasford, ELTS scores and ECOG between the two groups. The median time to initiate imatinib treatment was 23.5 (1- 156) days for Glivec group and 13 (1- 51) days generic group (p< 0.05). But the late onset of the treatment was not associated with treatment failure or death. The median follow up was 119.8 (3.7- 250.5) months for Glivec group and 43.6 (2- 150) months for generic groups, respectively (p< 0.05). This difference might be explained by the fact that Glivec has been on the market for about two decades. Similar rates of grade> 2 hematological and non- hematological toxicity were seen in Glivec (4.9 %) and generic groups (3.3 %), respectively (p> 0.05). The rates of treatment failure at 3 months were significantly higher in generic formulation (6.7 %) group than Glivec (2.9 %) group (p< 0.05). Also, the rates of treatment failure at 6 months were significantly higher in generic formulation (3.3 %) group than Glivec (0.9 %) group (p< 0.05). Optimal molecular response rate at 3 months was 76.5 % (n=78) for Glivec and 32.2 % (n=29) for generic groups (p< 0.001). Also, optimal molecular response rate at 6 months was 69.6 % (n=71) for Glivec and 45.6 % (n=41) for generic groups (p= 0.01). Median EFS was found significantly higher for Glivec group compared to generic group (168 mos (95% CI: 159-177 mos) vs 74.6 mos (95% CI: 56-93); p<0.001) (Figure).
Conclusion: We found that complete hematological response rates at 3 and 6 months were similar in both groups, but in early phase of treatment the optimal response rates of Glivec group was statistical significantly higher than generic group. Generic group presented with a lower rate of optimal response at 3 months but 13.4 % improvement in optimal response rates was observed at six months. No significant difference in safety concerns was observed between the groups. We recommend that these results from single center should be clarified in a prospective, randomized study including larger population.
Özcan:AbbVie: Other: Travel support, Research Funding; MSD: Research Funding; Novartis: Research Funding; Amgen: Honoraria, Other: Travel support; BMS: Other: Travel support; Jazz: Other: Travel support; Sanofi: Other: Travel support; Abdi Ibrahim: Other: Travel support; Janssen: Other: Travel support, Research Funding; Bayer: Research Funding; Celgene Corporation: Research Funding, Travel support; Takeda: Honoraria, Other: Travel support, Research Funding; Archigen: Research Funding; Roche: Other: Travel support, Research Funding. Beksac:Celgene: Speakers Bureau; Janssen: Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.