Background: Bosutinib is approved at a starting dose of 500 mg once daily (QD) in patients with Philadelphia chromosome-positive chronic myeloid leukemia (CML) who are resistant or intolerant to prior treatment and at a starting dose of 400 mg QD in newly diagnosed patients with chronic phase (CP) CML. Approval of bosutinib after prior therapy was based on a phase 1/2 study in patients previously treated with imatinib ± dasatinib and/or nilotinib. After long-term follow-up (≥4 years), durable responses and maintenance of health-related quality of life (HRQoL) were seen in patients after prior imatinib (CP CML second-line [CP2L] cohort [n=284]) or prior imatinib + dasatinib and/or nilotinib (CP CML third/fourth-line [CP3L/CP4L] cohort [n=115/4]). As a post-authorization commitment to the European Medicines Agency, the BYOND study is providing additional safety and efficacy data for bosutinib in patients with CML after failure of prior tyrosine kinase inhibitor (TKI) treatment. Cumulative confirmed major cytogenetic response rate by 1 year (primary endpoint; not powered) in evaluable patients with CP CML was 75.8% after 1 or 2 prior TKIs (n=99) and 62.2% after 3 prior TKIs (n=45). Cumulative complete cytogenetic response rate anytime on treatment was 86.0%, 83.9%, and 73.3% in the CP2L, CP3L, and CP4L cohorts, respectively. Patients had high rates of molecular responses across all lines of treatment. Evaluation of HRQoL through patient-reported outcome (PRO) measures is an exploratory objective of BYOND.
Methods: BYOND (NCT02228382) is an ongoing, phase 4, single-arm, open-label study of bosutinib at a starting dose of 500 mg QD in patients with CML and resistance/intolerance to prior treatment. At baseline and during treatment, patients were asked to complete the Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu v4) instrument, a 44-item, valid assessment of HRQoL in patients with leukemia. Each item was scored on a scale from 0 to 4, with higher scores indicating better HRQoL. Changes in HRQoL that are clinically meaningful to a patient have been defined as the minimal important difference (MID) for most FACT-Leu domains. We report PRO results at Month 12 of bosutinib treatment in the CP CML cohorts; for comparison, we present PRO data at Month 12 from the CP CML cohorts of the phase 1/2 study of bosutinib in previously treated patients.
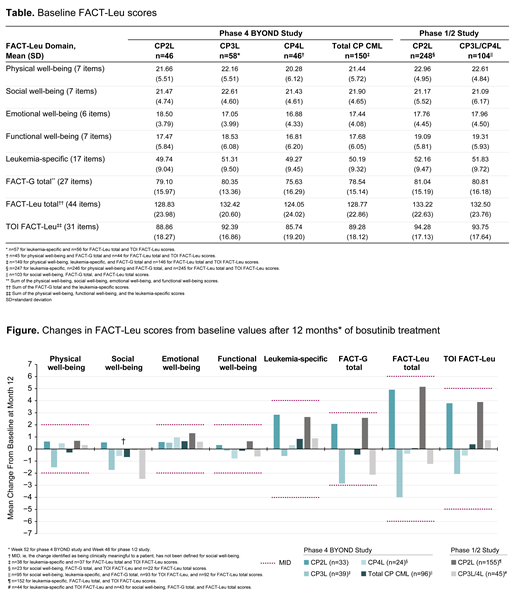
Results: At baseline, most FACT-Leu scores were similar (<5% difference) in the CP2L and CP3L cohorts of the BYOND study (Table); social and functional well-being scores were lower and the emotional well-being score was higher in the CP2L cohort. Baseline FACT-Leu scores were lower in the CP4L cohort, with >5% differences seen for physical and emotional well-being compared with the CP2L cohort, and for physical, social, and functional well-being, FACT-General (FACT-G) total, FACT-Leu total, and trial outcome index (TOI) FACT-Leu compared with the CP3L cohort. At Month 12, no mean change in a FACT-Leu domain score met the MID (Figure), indicating preservation of baseline HRQoL across all cohorts. Mean changes in FACT-Leu scores from baseline to Month 12 were similar in the CP2L cohorts of the BYOND study and the phase 1/2 study. HRQoL trends were also generally similar in the CP3L cohort of BYOND and the CP3L/4L cohort of the phase 1/2 study, in which 97% of patients received third-line bosutinib.
Conclusions: HRQoL was maintained from baseline in patients with CP CML following 12 months of bosutinib treatment in the BYOND study. HRQoL changes at Month 12 were comparable to those observed in previously treated patients in the initial phase 1/2 study of bosutinib, wherein long-term efficacy and HRQoL stability were subsequently reported. In addition, FACT-G scores in the BYOND study were consistent with those previously reported for general populations as well as patients with various cancers. Maintenance of HRQoL is important for patients with CP CML who potentially will receive lifelong TKI treatment, and the PRO results from BYOND suggest bosutinib is a well-tolerated treatment option, thus providing further support for its use in patients with CP CML resistant/intolerant to prior TKIs. Relationships between molecular response and HRQoL in the BYOND study are being explored.
Brümmendorf:University Hospital of the RWTH Aachen: Employment; Merck: Consultancy; Ariad: Consultancy; Pfizer: Consultancy, Research Funding; Janssen: Consultancy; Novartis: Consultancy, Research Funding. Gambacorti-Passerini:Bristol-Meyers Squibb: Consultancy; Pfizer: Honoraria, Research Funding. Abboud:Jazz Pharma: Speakers Bureau; Novartis: Other: Member on an entity's Board of Directors or advisory committees (Ended 12/30/2017), Research Funding; Agios: Other: Member on an entity's Board of Directors or advisory committees (Ended 12/30/2017); Tetraphase Pharmaceuticals: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; NKarta: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Bayer: Consultancy, Honoraria. Watts:Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Rosti:BMS: Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Russell-Smith:Pfizer: Employment, Equity Ownership. Viqueira:Pfizer Inc: Employment, Equity Ownership. Reisman:Pfizer Inc: Employment, Equity Ownership. Giles:Actuate Therapeutics Inc: Employment; Epigene Therapeutics Inc: Consultancy, Other: leadership, stock/other ownership ; Novartis: Consultancy. Hochhaus:MSD: Research Funding; Novartis: Research Funding; BMS: Research Funding; Incyte: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.