Background:
Patients with polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) are at increased risk of thrombosis. These myeloproliferative neoplasm (MPN) patients are historically treated with vitamin K antagonists (VKAs) to prevent recurrent thromboembolic events. Direct oral anticoagulants (DOACs) are novel anticoagulants that are being increasingly used in MPNs without robust evidence. We hypothesize that DOAC and VKA therapy have similar efficacy and safety in MPNs with a comparable incidence of recurrent thromboembolic and bleeding events.
Methods:
Using Looking Glass®, an interactive software that integrates demographic and clinical datasets for the purpose of clinical research, we identified MPN patients who were on systemic anticoagulation (AC) therapy for a documented arterial or venous thrombotic event between 1/1/2014 and 4/1/2019. Medical charts were reviewed to obtain patient demographics, MPN subtype, mutation status, date of MPN diagnosis, date and site of index thrombosis, date of AC initiation, last documented date of therapy, date and site of recurrent thrombosis, changes in therapy; date and type of major bleeding (MB) and clinically relevant non-major bleeding (CRNMB) events on AC. Primary outcome was incidence of recurrent thrombotic events while on AC. Secondary outcome was the incidence of MB and CRNMB events. Statistical analyses were performed using two-sided t-tests with α =0.05 for continuous variables, chi-square for categorical variables with samples size >10 and Fisher's exact tests for categorical variables with sample size <10.
Results:
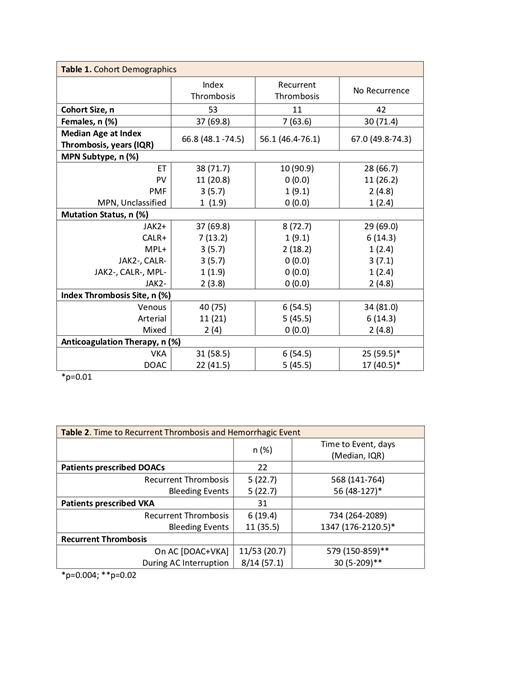
We identified 53 patients that met the study criteria. Data on demographics, MPN subtype, mutation status, index thrombosis site, and type of AC are reported in Table 1. The majority of the patients in our cohort were females (69.8%), had a diagnosis of ET (71.7%), were JAK2V617F mutated (69.8%), and had a venous index event (75%). Median age for the entire cohort was 66.8 years (IQR 48.1-74.5) while the median age for those who had a recurrent thrombotic event on AC was 56.1 (IQR 46.4-76.1). If patients were switched from one anticoagulant to another, only the first treatment was included in the analysis. 31 (58.5%) of patients were on VKA therapy while 22 (41.5%) were on DOAC therapy. Median time of follow up was 227 and 451 days for DOACs and VKA respectively, (p = 0.01).
Rates of thrombosis and bleeding were comparable within anticoagulation class. 5/22 (22.7%) and 6/31 (19.4%) of patients prescribed DOACs and VKA respectively developed recurrent thrombotic events. Median time to recurrent thrombotic event for DOACs as compared to VKA group was 568 vs 734 days (p = 0.29). 5/22 (22.7%) of those prescribed DOACs had bleeding events, 1 MB and 4 CRNMB. 11/31 (35.5%) of those prescribed VKA had bleeding events, 2 MB and 9 CRNMB. Median time to bleeding event for DOACs as compared to VKA was 56 vs 1347 days (p = 0.004). For the 53 patients on any anticoagulation, 11 (20.7%) patients had recurrent events while on AC (DOAC and VKA). In contrast, of the 14 patients who had AC therapy interrupted for any reason, 8 (57.1%) developed recurrent thrombotic events (p = 0.02). Table 2 summarizes these findings.
Conclusion:
We conducted a retrospective chart review study comparing the incidence of recurrent thromboembolic events on DOAC and VKA therapy. This is the largest study to date of MPN patients treated with DOACs. Based on our data, it appears that patients treated with DOACs have a comparable incidence of recurrent thrombosis and hemorrhagic events when compared to VKA therapy, although our study suggests that bleeding events may occur sooner in patients treated with DOACs. A large percentage of people with interruptions in their AC developed recurrent thrombotic events. Our data further emphasize the profound pro-thrombotic nature of MPN and therefore the need for uninterrupted anticoagulation therapy. Overall, given the comparable incidence of thrombosis and hemorrhage, DOAC therapy appears to be similar to VKA therapy in management of thrombotic complications of MPNs. The limitations of our study were as expected: a small sample size and significantly shorter median time for DOAC follow up as compared to VKA. Future, prospective large-scale studies are needed to confirm the safety and efficacy of DOAC therapy in MPNs.
Kushnir:Janssen Pharmaceuticals: Research Funding. Billett:Bayer Pharmaceuticals: Research Funding; Janssen Pharmaceuticals: Research Funding; Albert Einstein College of Medicine: Patents & Royalties: Patent application pending for NETs AI software.
Author notes
Asterisk with author names denotes non-ASH members.