Introduction
Although the median (med) age at diagnosis of CLL is 72 years (yrs), 11% are less than 55 yrs of age (SEER database). For this significant proportion of younger patients (pts), there exists a paucity of published data, particularly with regards to long-term follow up and safety. In recent years, single-centre retrospective analyses of younger CLL pts have been published (Parikh et al. 2015, Getta et al. 2018.) but this is the first study using data from international prospective randomised multi-centre trials, representing a large and diverse cohort of pts.
Methods
We performed a pooled analysis of pts receiving first-line treatment in seven phase II or III clinical trials conducted by the GCLLSG. This includes two phase III trials for early stage (CLL1 and CLL7), four phase III trials for advanced stages (CLL4, CLL8, CLL10 and CLL11) and one phase II trial (CLL2M). All trials evaluated chemotherapy or chemo-immunotherapy (CIT). Only patients who received treatment were included in this analysis. Using clinical and laboratory data, we compared baseline characteristics, treatments, efficacy and causes of death of pts in the age groups of ≤ 55 yrs and > 55 yrs. An age cut-off of 55 yrs has been selected based on similar historical studies. Further subgroup analyses were performed; (i) separating younger (≤ 55 yrs) pts into > 45 - ≤ 55 yrs and ≤ 45 yrs and (ii) pts with advanced stage CLL (including CLL4, CLL8, CLL10, CLL11 and CLL2M). Time to event was analysed by Kaplan-Meier method and hazard ratios (HR) with confidence intervals (CI) calculated using Cox regression model.
Results
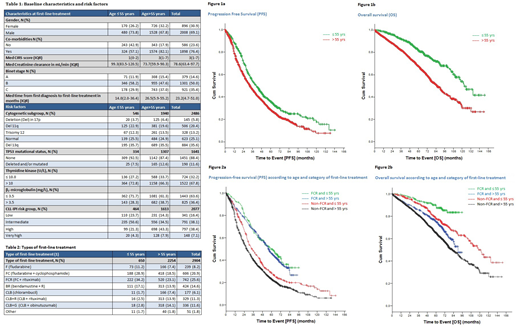
In total, 2904 pts were included in this meta-analysis, 650 (22.4%) pts were younger (≤ 55 yrs) and 2254 (77.6%) were older (> 55 yrs), the med age being 63 yrs with a med observation period of 67.7 months. Baseline characteristics are shown in table 1 and frontline therapies in table 2. All six cycles of therapy were completed in 80.5% of the younger pts and 75.3% of the older pts.
The best overall response (CR/PR) was 92.8% for younger pts and 85.8% for older pts. 33.8% and 25.7% achieved a CR respectively. Minimal residual disease negativity (< 10-4) was achieved in 22.3% and 16.2% respectively, with missing results in 56.4% of total, as per intention to treat approach. The med duration of response was 25.3 months in older pts as compared to 36.1 in younger pts (HR: 1.353, 95% CI: 1.205-1.520). Med progression-free survival (PFS) was 32.7 months in older pts as compared to 41.3 in younger pts (HR: 1.292, 95% CI: 1.160-1.438) respectively (figure 1a), with similar results in the pts with advanced disease. No significant difference in PFS was found within the two groups less than 55 yrs of age ( > 45 - ≤ 55 yrs and ≤ 45 yrs). Med time to next treatment (TTNT) was similar between the ≤ 55 yrs cohort and > 55 yrs (62.5 month and 59.1 months). Med overall survival (OS) was 85.8 months in the > 55 aged pts as compared to 122.7 months in the ≤ 55 year old pts (HR: 2.023, 95% CI: 1.698-2.411) (figure 1b), with similar results in the advanced CLL pts. PFS and OS for younger and older pts, was significantly better, when FCR was administered in comparison to all other treatments, including fludarabine (F), F + cyclophosphamide(FC), bendamustine and rituximab (BR), chlorambucil (CLB) alone and CLB + R, CLB + obinutuzumab (G) ( figures 2a and 2b). Death, as determined by investigator, was CLL-related in 50.3% of younger pts and 46% of older pts. In the younger group; CLL-related death was due to progressive disease (PD) in 46.7%, infection 40% and Richter transformation (RT) 12%. Amongst the older pts, death was attributed to PD in 62.2%, infection in 31% and RT in 6%. CLL-unrelated deaths (22.8%) occurred in younger pts; due to co-morbid conditions in 47.1%, secondary malignancy in 38.2% and adverse events (AEs) in 11.8%. In the older pts, Co-morbid conditions (39.1%), AEs (35.6%) and secondary malignancy (23.6%) resulted in the CLL-unrelated deaths.
Conclusions
This meta-analysis of younger CLL pts, demonstrated that being aged 55 yrs or younger has a significant impact on OS and PFS. While pts younger than 55 yrs respond better to therapy and show a longer PFS, they clearly suffer from an elevated rate of late complications. In this regards, a higher rate of death due to secondary malignancy in the younger pts is of particular interest. The data define the need for first-line treatments with reduced long-term toxicity and warrant long-term outcome analyses in younger pts treated with novel agents.
Cramer:mundipharma: Other: travel support; Roche: Honoraria, Other: travel support, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Gilead: Other: travel support, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Fink:Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Other: travel grants; Celgene: Research Funding. Fischer:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Other: travel grants. Goede:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants, speaker fees, Speakers Bureau; janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants, speaker fees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: speaker fees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Herling:Roche: Other: travel grants, Research Funding. Wendtner:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria, Research Funding; Janssen-CILAG: Consultancy, Honoraria, Research Funding; GILEAD Science: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Mundipharma: Honoraria, Research Funding. Hallek:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Eichhorst:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; ArQule: Membership on an entity's Board of Directors or advisory committees; BeiGene: Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.