Introduction: Response criteria in light-chain (AL) amyloidosis, revised in 2012, did not specify a depth of change in the pathologic involved FLC (iFLC) for either an amyloid complete response (aCR) or a very good partial response (VGPR) (JCO 2012;30:4541). The FLC ratio upon which an aCR is based is impacted if renal function deteriorates and if either the iFLC or uninvolved FLC (uFLC) become undetectable; moreover, the VGPR was based on the difference between iFLC and uFLC and not on the response in the iFLC. Emerging data have controverted the role of the FLC ratio, suggesting the need for a revised criteria, particularly since a new CD38-based treatment regimen may achieve iFLC levels <10mg/L in over 70% of patients and may often make the ratio incalculable (Abs #S875 24th European Hematology Association Congress;6/15/19). In a revisionist mode, we examined overall survival (OS) from diagnosis in patients achieving > VGPR as a function of baseline, treatment-related and iFLC response variables.
Patients and Methods: Patients with AL diagnosed between 2005-2017 with > VGPR after treatment were included in this IRB-approved retrospective study. Cox proportional-hazards regression analyses were used to assess the impact of baseline variables (gender, age at diagnosis, pathologic light-chain isotype, marrow plasma cells, iFLC, dFLC, and cardiac and renal stage), and also to assess the impact of treatment and iFLC-response related variables (types of initial therapy, number of courses of therapy [induction if needed+MEL SCT+consolidation = 1 course], exposure to daratumumab, achievement of aCR and VGPR, and iFLC response). iFLC responses were defined as levels <10, 10-20 or > 20mg/L. MedCalc 19.0.3 statistical software was used for all statistical analyses.
Results: One hundred and thirty-three patients met the criteria and were 78M/55W a median of 60.5 yo (range, 35-81) with AL-lambda type in 114 (86%) and marrows with a median 10% plasma cells (1-50). Median iFLC and dFLC were 135mg/L (29.4-9780) and 123mg/L (4-9770) respectively, and 89 patients (66%) had cardiac (stage II=49, III=33) and 87 (65%) renal involvement (stage II=55, III=16). Of baseline variables, only age and cardiac stage were significant predictors of OS.
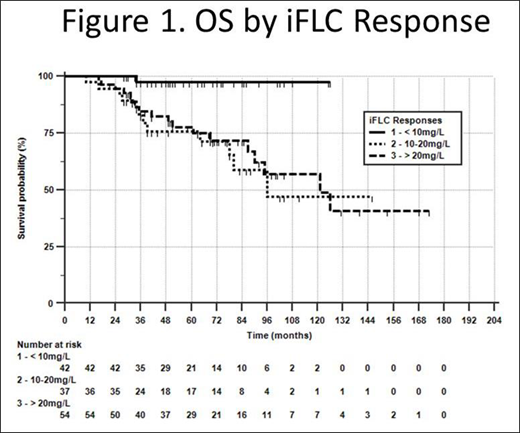
Ninety one (68%) had bortezomib-based initial therapy and 62 (47%) went to SCT+consolidation. Eighty-three (63%) received 2 courses of therapy and 14 (11%) received 3 courses; 7 received daratumumab in second-line therapy and 14 in the third-line. Eighty four (63%) achieved an aCR. With a median follow up > 5 years, of treatment and response related variables, only the iFLC response predicted OS (p<0.01). Log-rank (Kaplan-Meier) analysis showed that patients achieving iFLC <10mg/L had over 95% survival at 120 months, compared to those achieving iFLC 10-20mg/L or >20mg/L whose median OS were 96 and 121 months respectively (p<0.01) (Figure 1). We then compared patients in the iFLC groups. They did not differ by Mann-Whitney in age at diagnosis or by χ2 with respect to cardiac stage, types of initial therapy, number of courses of therapy, or exposure to daratumumab. However, the groups differed significantly by Mann-Whitney in baseline iFLC/dFLC [medians, 75/62 (<10), 212/192 (10-20), 227/190 (>20) (all comparisons, p<0.01)], and by χ2 in aCR rates [81% (<10), 67% (10-20) and 47% (>20) (p<0.01)].
Conclusions: Multiple variables impact OS in patients achieving > VGPR to therapy, including age, cardiac stage at diagnosis, and iFLC response. In this series, the optimal hematologic response is an iFLC <10mg/L and the major challenge to achieving that goal is the scale of FLC disease at diagnosis. Further studies are needed to validate a revision of the hematologic response criteria in AL in the modern era of monoclonal antibody therapy.
Comenzo:Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Caelum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Myself: Patents & Royalties: Patent 9593332, Pending 20170008966; Karyopharm: Research Funding; Prothena Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.