Background
The advent of new agents has contributed to the prolonged survival of multiple myeloma (MM) patients. From the Durie-Salmon staging system, international staging system (ISS) and recently, revised ISS (R-ISS) have been developed to better prognosticate the survival of MM. R-ISS includes chromosomal abnormalities (CA) detected by interphase fluorescent in situ hybridization (iFISH) and lactate dehydrogenase (LDH) in addition to the ISS. However, the R-ISS has still some limitations in the real clinical settings, as it has enrolled patients exclusively on clinical trials which includes more young patients (age < 65 years old), contains non-standardized iFISH results and has short median follow-up duration. The first-line drugs used in the patients could also affect the prognosis. Therefore, a more detailed and standardized but also convenient prognostic system is needed with clinical findings commonly observed in MM patients treated with new agents in the real clinical world.
Patient and Method
Adult patients who were confirmed multiple myeloma between January, 2011 to December, 2017 and patients who had received thalidomide and/or bortezomib or lenalidomide-based chemotherapy as a first-line treatment were included for the analysis. A total of 861 patients from 13 centers participating Korean multiple myeloma working party were analyzed. Baseline serum albumin, serum ß2-microglobulin, cytogenetics by iFISH, LDH (lactate dehydrogenase), ALC (absolute lymphocyte count), CRP (C-reactive protein) and ferritin were measured within 4 weeks before beginning the first line of chemotherapy. Each factors of age ≥ 65 years, serum ß2-microglobulin ≥ 5.5 mg/L, LDH > normal, cytogenetic high risk by FISH (del17p, t(4;14), t(14;16)), ALC < 1500/mm3, CRP ≥ 1.5 mg/dL, and ferritin ≥ 500 ng/mL were defined as abnormal findings, which were given 1 point (Table 1) and the sum of points were used to discriminate inflammatory factor-based staging system (IFBSS). IFBSS were defined as follows: Stage I (point 0), stage II (point 2-3), stage III (point 4-5), and stage IV (point 5-7). Overall survival (OS) was defined by the date of MM diagnosis to the date of death by any cause or follow-up loss.
Results
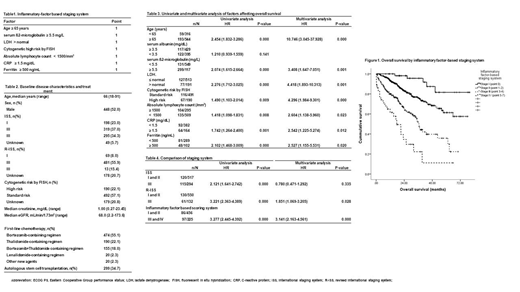
Baseline characteristics of the patients were as listed in Table 2. With a median follow-up duration of 22.70 months (range, 0.20-86.80 months), age ≥ 65 years, serum ß2-microglobulin ≥ 5.5 mg/L, LDH > normal, cytogenetic high risk by FISH (del17p, t(4;14), t(14;16)), ALC < 1500/mm3, CRP ≥ 1.5 mg/dL, and ferritin ≥ 500 ng/mL showed significant higher OS by univariate and multivariate analysis. Albumin < 3.5 mg/dL were excluded into the staging system due the unpredictability to OS in univariate analysis (Table 3). Study groups of inflammatory factor-based scoring system comprised of 61 patients in stage I, 395 patients in stage II, 189 patients in stage III and 36 patients in stage IV. The median OS were not reached in stage I and II, stage III and IV showed a median OS of 36.57 months (95%CI, 33.96-.39.18) and 17.60 months (95%CI, 9.16-.26.04). The OS according to the IFBSS showed significant differences (P < 0.001), which predicted OS better compared with conventional ISS and R-ISS (Table 4).
Conclusion
In the new agent era, inflammatory factors incorporated into the staging system can better discriminate prognosis of multiple myeloma patients compared to the ISS or R-ISS. Validation of this finding in a larger cohort is needed to expand usage of this new staging system.
Yoon:Novartis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Kyowa Hako Kirin: Research Funding; Janssen: Consultancy; Yuhan Pharma: Research Funding; Genentech, Inc.: Research Funding; MSD: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.