Background: B cell maturation antigen (BCMA) is a potential therapeutic target in multiple myeloma. CT053 CAR-BCMA, which is a BCMA-specific chimeric antigen receptor (CAR) T cell, consists of autologous T cells genetically modified with a second-generation CAR incorporating a fully human anti-BCMA single chain fragment variant, a 4-1BB co-stimulatory domain and a CD3-zeta signaling domain. CT053 was studied in a single-arm, open-label phase I program (NCT03716856, NCT03302403, and NCT03380039) in eastern China.
Methods: This investigation is a 3-site study in adult subjects with relapsed/refractory multiple myeloma (rrMM) who had received at least 2 prior myeloma regimens. Subjects received one cycle of CT053 CAR-BCMA after fludarabine/cyclophosphamide infusion. The primary objective was the assessment of subject safety. The secondary objectives were pharmacokinetics and efficacy. Efficacy was assessed according to IMWG 2016 criteria. The data cutoff date was June 30, 2019.
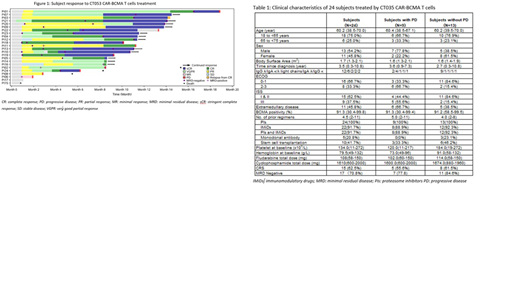
Results: A total of 24 subjects with median age of 60.1 years (range, 38.5-69.9) were enrolled from Sep 10, 2017 to Sep 22, 2018 (Table 1). The subjects had a median of 4.5 (range, 2-11) prior regimens of therapy, and 41.7% (10/24) underwent autologous stem cell transplantation. At baseline, eleven out of 24 subjects (45.8%) had concomitant extramedullary involvement. Eight subjects (33.3%) had ECOG score 2-3, and 9 subjects (37.5%) reported ISS grade III. All subjects received lymphodepletion preconditioning of fludarabine/cyclophosphamide for 2-4 days, followed by one cycle of CT053 at a dose of 1.5 x 108 T cells except 3 subjects who received 0.5 x 108, 1 x 108, and 1.8 x 108 cells, respectively.
The overall response rate was 87.5% (21/24) including 79.2% (19/24) with complete responses or stringent complete response (5 CR, 14 sCR). As shown in Figure 1, P1 who received the lowest dose of 0.5 x 108 experienced partial response (PR) at M1 and very good partial response (VGPR) at M2. P1 then converted to CR at M14 and sCR at M16. Among 20 subjects who underwent the evaluation of minimal residual disease (MRD) status, 17 achieved MRD-negative (≤10−4 nucleated cells) and reported a tumor response (17 CR/sCR). In 13 subjects with ongoing CR/sCR, the median follow-up after CT053 infusion was 383 days (range, 301-467). Nine subjects progressed with median progression-free period of 281 days (range, 57-573); among them, 5 progressed within 6-12 months, 1 at 13 months and 1 at 19 months. Compared to 13 subjects with persistent CR/sCR, the 9 progressed subjects were observed to have a higher percentage of ECOG score 2-3 (66.7% vs 15.4%), ISS Grade III (55.6% vs 15.4%), and concomitant extramedullary diseases (66.7% vs 38.5%) and a decreased hemoglobin (70g/L vs 92g/L) at baseline. Three subjects died of disease progression at the time of analysis.
Hematologic toxic effects were the most common treatment-related adverse events of grade (G) 3 or higher, including white blood cell count decreased (87.5%), neutrophil count decreased (66.7%), lymphocyte count decreased (79.2%) and thrombocytopenia (25%). No dose limiting toxicities were observed. Low-grade cytokine release syndrome (CRS) was reported in 15 of 24 (62.5%) subjects. All CRS events (3 G1, 12 G2) recovered within 2-8 days; among them 8 received tocilizumab. Three subjects (12.5%) had neurotoxicity (2 G1, 1 reversible G3). One subject died of bone marrow failure and neutropenic infection.
CAR-BCMA T cell expansion was detected as early as Day 1-7 after infusion and reached peak values on Day 7-21 with the highest at 4.5×105 copies/µg genomic DNA. Median T cell persistence was 172 days. The longest persistence of CAR-BCMA copies was measured at 341 days and continues.
Conclusion: This study demonstrated that CT053 had an excellent efficacy and a good safety profile in subjects with rrMM.
Li:CARsgen Therapeutics Co. Ltd: Employment, Equity Ownership. Xiao:CARsgen Therapeutics Co. Ltd: Employment. WANG:CARsgen Therapeutics Co. Ltd: Employment. Yuan:CARsgen Therapeutics Co. Ltd: Employment. Ma:CARsgen Therapeutics Co. Ltd: Employment.
Author notes
Asterisk with author names denotes non-ASH members.