Introduction:
Clotting factor replacement therapy in the management of persons with hemophilia A (PwHA) is typically dosed using the following weight-based formula:
Factor VIII (FVIII) Dose = Body weight (BW) in kg X desired FVIII increase (%)
2
This equation is based upon a FVIII in vivo recovery (IVR) of 2, which assumes that each FVIII unit per kg of BW infused will increase the circulating FVIII concentration by 2%. There have been a few studies demonstrating that IVR increases with increasing BW. A pharmacokinetic study performed using recombinant FVIII concentrate for pediatric PwHA <6 years of age found that rising body mass index (BMI) resulted in a linear increase in IVR [Blanchette VS, et al. J Thromb Haemost 2008; 6: 1319-26]. Using data from eight pharmacokinetic clinical trials evaluating three recombinant FVIII concentrates in adult PwHA, Henrard et al. demonstrated that the IVR for an adult cohort consisting of 201 PwHA was 2.16. However, when these patients were stratified based on BMI: <20.3 kg/m2, between 20.3 and 29.5 kg/m2, and >29.6 kg/m2, each group had a significantly different and increasing IVR of 1.60, 2.14 and 2.70, respectively [Henrard S, et al. Haematologica 2013; 98: 1481-6]. As there are no guidelines for dosing of factor replacement therapy for obese PwHA, these studies suggest that ideal body weight (IBW) could be considered instead of actual body weight (ABW) in the dose calculations. With the prevalence of obesity in the hemophilia population now approaching that of the general population, dosing using IBW has potential economic ramifications [Wong TE, et al. Am J Prev Med 2011; 41: S369-75, Majumdar S, et al. Haemophilia 2011; 17: 717-8].
Objectives:
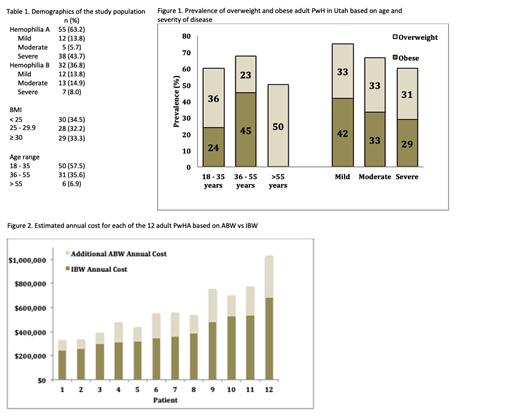
1) To estimate the prevalence of overweight and obese adult persons with hemophilia (PwH) in the state of Utah;
2) To estimate the cost savings for annual prophylactic clotting factor prescriptions if they were to be written using IBW vs. ABW for adult PwHA that are obese in the state of Utah.
Methods: Adult PwH (≥18 years of age) who have been seen at least once in the past 3 years (January 1, 2016 to December 31, 2018) were identified from the Utah Center for Bleeding & Clotting Disorders Registry. The following information was collected: Demographics; type of hemophilia; severity of hemophilia; height; weight; BMI. If BMI was not available, this was calculated based on the following equation, BMI = kilograms/meters2. Overweight was defined as a BMI 25 - 29.9 kg/m2. Obese was defined as a BMI ≥ 30 kg/m2.
As there is limited data on the relationship between increasing BMI and IVR for factor IX concentrates, persons with hemophilia B were excluded in our cost savings analysis. For PwHA on clotting factor prophylaxis who were obese, the IBW was calculated using their height and the following equation for men:
IBW (in kg) = 50 + 2.3 kg per each inch over 5 feet
Using the prophylaxis schedule for each obese PwHA, an estimate of the annual cost was calculated using Wholesale Acquisition Cost and compared using both ABW and IBW.
Results:
A total of 87 adult PwH were seen with documented measurements of height and weight within the past 3 years. The patient characteristics are presented in Table 1. The mean and median age was 37.1 and 34 years, respectively (range: 19 - 75 years). The mean value for BMI was 28.1 and the median was 27.2 (range 17.3 - 46.0). Two patients had class III obesity (BMI ≥40 kg/m2). Almost two-thirds of adult PwH were overweight or obese (58/87; 65.5%). The prevalence of overweight and obese adult PwH in Utah based on age and severity of disease is shown in Figure 1.
The estimated annual cost for each of the 12 adult obese PwHA based on their ABW vs IBW is shown in Figure 2. The difference in the total annual cost of treatment for the 12 adult PwHA on prophylaxis factor replacement therapy would be reduced by a total of roughly $2,100,000 if IBW was used rather than ABW; an average savings of $175,000 per adult PwHA per year.
Conclusion:
The relatively high prevalence of overweight and obesity in the Utah hemophilic population is similar to what has been reported in other states across the United States. The use of IBW in dose calculations has significant economic ramifıcations. Further studies performing individualized pharmacokinetic analysis for PwHA using IBW dosing must be coupled with close clinical monitoring to ensure safety of this dosing strategy before it can be adopted into routine clinical practice.
Rodgers:Pfizer: Consultancy; Octapharma: Consultancy; Novartis: Consultancy; Sanofi: Consultancy; AstraZeneca: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract