Background. We recently reported that Circulating Endothelial Cell (CEC) count changes represent a promising marker to monitor endothelial damage in patients undergoing allogeneic hematopoietic stem cell transplant (allo-HSCT), potentially becoming a valuable tool in the diagnostic definition of GVHD. Besides confirming an increase of CEC counts at GVHD onset, we repeatedly documented at time of engraftment statistically significant higher numbers of CEC in patients who will not manifest GVHD in comparison to patients in which GVHD will be diagnosed (Transplantation 2014,98:706-12; Bone Marrow Transplantation 2017,52:1637-42; Scientific Reports 2019,9:1-12). Recent knowledges in organ transplant pointed out that endothelial cells from the grafted organ, besides being a continuous source of alloantigens, can downregulate alloreactivity exerting tolerogenic responses. By inference to the allo-HSCT field, it could be envisaged that presence of donor CEC could induce protective effects on alloreactivity.
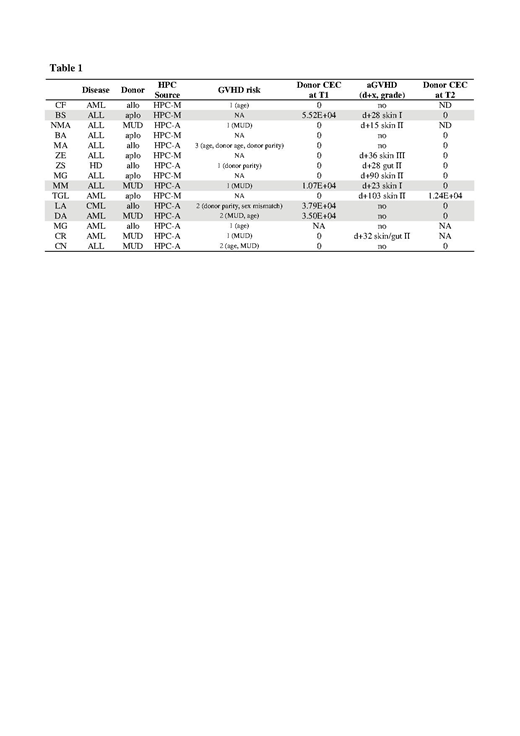
Methods. We planned a study to test the hypothesis that at time of engraftment, CEC present in peripheral blood (PB), besides coming from cells shedding from patient vasculature, could partly belong to donor, originating from the cellular graft. Therefore, in an exploratory set, we performed FISH analysis on flowcytometry-sorted CEC (CD45neg/CD34bright/CD146pos, Lyotube #623920, BD Biosciences) (n=3) and on whole PB derived culture-expanded CEC (n=3) (EGM-2 BulletKit, Lonza), obtained at engraftment in sex-mismatched allo-HSCT. In the confirmatory set (n=15), single CEC were recovered from PB, at engraftment (T1) and at 90 days (T2) after allo-HSCT, through the DEPArrayTM technology (Menarini Silicon Biosystems), after preliminary bulk separation step carried out with the CellSearch® System. Single recovered CEC was whole genome amplified (Ampli1™ WGA Kit) and short tandem repeat (STR) profile determined (Ampli 1TM STR kit) on each single CEC. To confirm host/donor origin, single CEC STR profile was compared to that determined on patient and donor cells before allo-HSCT. Moreover, donor CEC presence was evaluated by CISH analysis on formaline fixed and paraffin-embedded biopsy sections obtained at least three months after sex mismatched allo-HSCT.
Results. By positive findings of the exploratory set, we proved, at the single cell level in the confirmatory set, the presence of donor CEC at engraftment (T1) in 4 out of 15 patients (Table 1). Of them, 2 did not manifested GVHD, despite a GVHD risk score of 2, and the other 2 presented GVHD grade I. On the contrary, among the 10 patients in whom no donor CEC were detected, 6 experienced GVHD grade II-III, while 4 did not manifested GVHD, despite a 1-3 GVHD risk score.
Conclusions. Our data represent the proof of principle that donor CEC may flow in host PB early on from hematopoietic recovery and seldom persist thereafter at steady-state conditions, being potentially embedded in host vascular wall. These puzzling findings suggest that neovascularization takes place in parallel with hematopoietic engraftment and could provide further clues on shedding light on tissue tolerance in the context of GVHD, opening up paradoxical scenarios on the protective role potentially played by donor CEC.
Fontana:Menarini Silicon Biosystem: Employment. Rotta:BD Biosciences Italia: Employment. Manaresi:Menarini SIlicon Biosystem: Employment, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.