Background: Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare but life-threatening thrombotic microangiopathy, with an untreated mortality rate of >90%. Prompt treatment with therapeutic plasma exchange (TPE) and immunosuppression improves outcomes in patients with aTTP, but 10-20% of patients still die acutely from this disease. The aim of this analysis was to describe in more detail the characteristics and disease courses of the patients who died during the caplacizumab clinical development program.
Methods: Patient narratives on all deaths occurring during the phase 2 TITAN and phase 3 HERCULES studies were extracted.
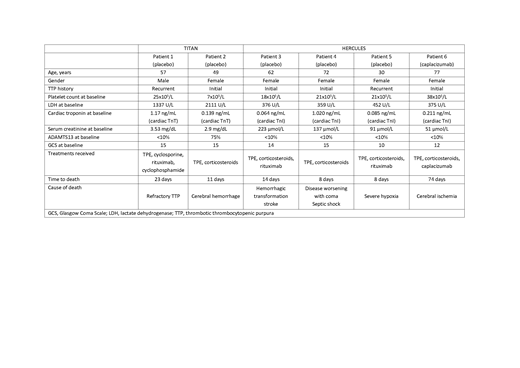
Results: In the overall study periods, a total of 6 patients died, 2 patients enrolled in TITAN (Patients 1 and 2) and 4 patients enrolled in HERCULES (Patients 3-6). Five patients received placebo, while 1 (Patient 6) received caplacizumab. Demographics and baseline disease characteristics are summarized in Table 1. The patient narratives are provided below.
Patient 1 (placebo) was a 57-year-old male, with a recurrent episode of aTTP. Baseline platelet count was 25 x109/L and ADAMTS13 activity <10%. The patient was treated with daily TPE and cyclosporine. Because of lack of response to TPE, treatment was intensified (increased plasma volume exchanged, and rituximab and cyclophosphamide initiated). Still, the patient's clinical condition declined with rising lactate dehydrogenase (LDH) values and persistent thrombocytopenia, with a fatal outcome on Day 23.
Patient 2 (placebo) was a 49-year-old female, with an initial presumed aTTP episode. Baseline platelet count was 7 x109/L and baseline ADAMTS13 activity was 75%. The patient did not respond to therapy (TPE and corticosteroids), with platelet counts remaining below 35 x109/L over the whole period. On Day 10 of the study, the patient experienced a cerebral hemorrhage, for which study drug treatment was permanently discontinued. The patient was intubated and died the next day of cerebral hemorrhage.
Patient 3 (placebo) was a 62-year-old female with an initial aTTP episode. Baseline platelet count was 18 x109/L and ADAMTS13 activity <10%. The patient was treated initially with daily TPE and corticosteroids. On Day 2 the patient suffered from a massive ischemic stroke with hemorrhagic transformation; TPE was interrupted for 2 days and restarted thereafter. The platelet count improved to 104 x109/L, but fell to 44 x109/L on Day 6. Rituximab was added on Day 8, however, the patient died on Day 14 from the consequences of the stroke.
Patient 4 (placebo) was a 72-year-old female with an initial aTTP episode. Baseline platelet count was 21 x109/L and baseline ADAMTS13 activity was <10%. Despite an initial (partial) platelet count response, the disease worsened with coma starting on study Day 6 and severe septic shock 2 days later. The patient died on Day 8 due to these events.
Patient 5 (placebo) was a 30-year-old female enrolled with her third aTTP episode. Baseline platelet count was 21 x109/L and ADAMTS13 activity <10%. The patient did not respond to therapy (TPE, corticosteroids, rituximab), with platelet count remaining below 30 x109/L over the whole period. Respiratory failure was reported on study Day 8, likely due to alveolar hemorrhage, with fatal outcome the same day; an autopsy was not performed.
Patient 6 (caplacizumab) was a 77-year-old female with her initial aTTP episode. Baseline platelet count was 38 x109/L and ADAMTS13 activity <10%, and was treated with TPE, corticosteroids and caplacizumab. Following daily TPE (for 36 days without tapering), the patient completed 30 days' treatment with caplacizumab, maintaining normal platelet counts during that period. ADAMTS13 activity at the end of study drug treatment was 62%. On follow-up Day 5, the patient was hospitalized with severe cerebral ischemia leading to death 3 days later; the event was considered not related to the study drug.
Conclusion: Although the use of TPE and immunosuppression reduces mortality in patients with aTTP, the disease is still associated with a substantial risk of mortality. The fact that all 5 immediate deaths occurred in the placebo arm suggests that the use of caplacizumab has the potential to reduce acute mortality in patients with aTTP.
Table 1. Baseline demographics and disease characteristics.
Cataland:Ablynx/Sanofi: Consultancy, Research Funding; Alexion: Consultancy, Research Funding. Scully:Alexion: Consultancy; Ablynx/Sanofi: Consultancy; Novartis: Consultancy; Shire/Takeda: Consultancy; Shire: Research Funding. Peyvandi:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Honoraria; Kedrion: Honoraria; Alnylam: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Octapharma: Research Funding. Knoebl:Roche: Consultancy; Shire/Takeda: Consultancy; Novo-Nordisk: Consultancy, Research Funding; CSL-Behring: Consultancy; Ablynx/Sanofi: Consultancy. Kremer Hovinga:Ablynx/Sanofi: Consultancy, Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Shire: Consultancy, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology), Research Funding; CSL-Behring: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Roche: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology); Siemens: Honoraria, Other: Honoraria go to employer (Insel Gruppe AG, Department of Hematology). Coppo:Ablynx/Sanofi: Consultancy; Alexion: Consultancy; Shire: Consultancy. Metjian:Genentech: Consultancy, Research Funding; AblynxNV/Sanofi: Consultancy, Research Funding. De La Rubia:Takeda: Consultancy; AMGEN: Consultancy; Janssen: Consultancy; Celgene Corporation: Consultancy; AbbVie: Consultancy. Pavenski:Ablynx: Honoraria, Research Funding; Bioverativ: Research Funding; Shire: Honoraria; Octapharma: Research Funding; Alexion: Honoraria, Research Funding. De Winter:Ablynx, a Sanofi company: Employment. Callewaert:Sanofi (formerly employed by Ablynx, a Sanofi company): Employment.
Author notes
Asterisk with author names denotes non-ASH members.