Introduction
While effective at treating and preventing thrombosis, modern forms of anticoagulation universally increase the risk of hemorrhage. Data suggests that factors FXI and FXII could serve as druggable targets to provide anticoagulation without increasing the risk of bleeding. The purpose of this systematic review is to evaluate the safety of novel drugs targeting FXI and FXII in human clinical trials.
Methods
We performed a search in Ovid MEDLINE to identify published manuscripts describing administration of contact pathway-inhibiting drugs to humans. All human clinical trials evaluating a drug specific to FXI or FXII were included. Outcomes of interest collected for analysis included primarily bleeding events of any type, total adverse events (AEs)as well as other treatment-emergent adverse events (TEAE) and treatment-related adverse events (TRAE).
Results
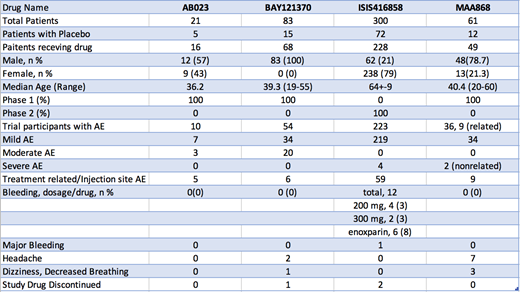
A total of 338 published articles were identified from the original search. After screening, one phase 2 study of an antisense oligonucleotide (ISIS 416858) and three phase 1 trials of monoclonal antibodies (AB023, BAY1213790, MAA868) were included. A total of 465 patients across these 4 clinical trials were included. No patients experienced spontaneous bleeding. Postoperative bleeding occurred in 3% of patients treated with ISIS 416858 at each dose level and one patient experienced major bleeding. For comparison an 8% postoperative bleeding rate was seen in the arm treated with prophylactic low molecular weight heparin (LMWH).
AB023, a monoclonal antibody inhibiting FXIIa-mediated activation of FXI to FXIa, was examined in a phase 1, dose-escalation trial in 21 healthy adults. Overall, there were no severe AEs. Minor AEs occurred in 10 of the 21 patients. 3 patients were deemed to possibly have TRAE. There was no statistical difference in bleeding time compared to placebo. activated thromboplastin time (aPTT) was prolonged for over a month after the highest dose level.
BAY1213790 is a monoclonal antibody targeting the enzymatic active site of FXIa, and was studied in a phase 1 dose escalation trial in 83 healthy men. There were no severe AEs. Of the 54 subjects experiencing AEs, 34 were mild and 20 were moderate. TRAE were reported in 6 subjects. One subject had an infusion reaction and another subject requested the infusion be stopped. APTT showed a dose-dependent increase while bleeding times also did not increase compared to placebo.
ISIS 416858 is an antisense oligonucleotide targeting FXI mRNA for degradation at its source within the hepatocyte. ISIS416858 was studied in a phase 2, randomized controlled trial in patients undergoing total knee arthroplasty. The safety and efficacy at preventing post-operative venous thromboembolism (VTE) was assessed at 200 mg or 300 mg doses compared to standard-of-care with LMWH. Bleeding occurred in 4 (3%), 2 (3%), and 6 (8%) patients in these three study groups, respectively. This was the only study drug where one patient experienced major bleeding. Mild AEs affected 219 subjects, with severe AEs occurring in 4 patients; only 2 discontinued ISIS416858. Patients in the 300 mg dose cohort of ISIS416858 experienced statistically fewer VTE events compared with LMWH; bleeding rates were numerically low compared to LMWH (3% vs 8%), though statistical significance was not reached.
MAA868 is a monoclonal antibody targeting both the zymogen and active forms of FXI at the enzymatic active site, and was studied in a phase 1, dose-escalation clinical trial. MAA868 is unique due to its subcutaneous route of administration. Of the total 61 patient cohort, 34 patients experienced AEs and 9 TRAE. Only 2 severe, unrelated AEs occurred after the trial: one fatal cardiac arrest after elective surgery, and a gunshot wound requiring urgent surgery. MAA868 resulted in prolonged FXI suppression for up to four weeks.
Conclusion
Contact pathway-inhibiting drugs show promise as novel anticoagulation strategies that could significantly reduce the risk of bleeding seen with traditional anticoagulants. Additional studies are needed to further develop these drugs to test their efficacy at targeting the coagulation pathway in common settings of high risk for VTE, or existing thrombosis. The paradigm that anticoagulants cause bleeding could be broken in the near future based on this promising clinical data on contact pathway inhibition.
Shatzel:Aronora, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.