The generation of hematopoietic stem and progenitor cells (HSPCs) from induced pluripotent cells (iPSCs) holds a great potential in development of cell therapies, modeling hematological malignancies, and testing new drugs. Hematopoiesis is regulated by bone marrow (BM) microenvironmental factors such as BM architecture, and cell-cell and cell-matrix interactions. The current methods for iPSC differentiation into HSPCs rely on cell-cell interaction (embryoid body formation and feeder cell co-cultures) or cell-matrix interaction (ECM coated dishes). Generation of a 3-dimensional (3D) culture environment attempts to capture both cell-cell and cell matrix interactions in studying the differentiation of the iPSCs. This study reports development of a 3D culture system for hematopoietic differentiation of iPSCs.
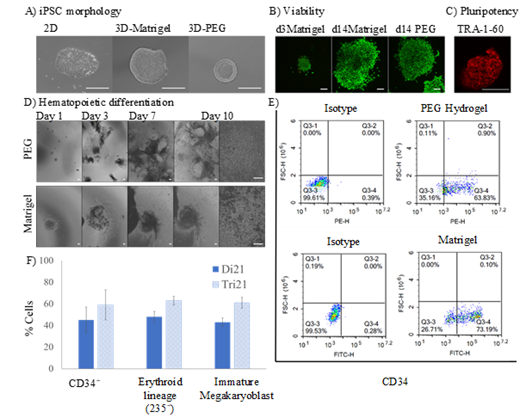
iPSC colonies were encapsulated in 3D Matrigel or polyethylene glycol (PEG) based hydrogels containing synthetic integrin binding peptide (GRGDSPC) and enzymatically degradable peptide (GGPQGIWGQGKG) and cultured in feeder-free cell culture and maintenance medium (mTeSR1™, Stem Cell Technology). There were notable morphological differences between the 3D encapsulated and 2D cultured iPSC colonies (Fig 1A). The 3D encapsulated colonies were more compact with a spheroid morphology in PEG compared to Matrigel, whereas colonies in 2D were more diffused. Viability of the encapsulated colonies was evaluated in situ using cytotoxicity kit. The 3D encapsulation did not have an adverse effect on the viability and growth of iPSCs (Fig 1B). The encapsulated iPSCs maintained the pluripotent phenotype in 3D as assessed by TRA-1-60 staining (Fig 1C).
To test the efficiency of these 3D model systems to generate HSPCs, the encapsulated iPSCs were subjected to hematopoietic differentiation using STEMdiff™ Hematopoietic Kit (Fig 1D). Following differentiation, the single cells were collected and immunophenotype analysis was performed by flow cytometry. Above 50% population stained positive for HSPC surface marker CD34 both in PEG hydrogel and Matrigel (Fig 1E).
To test the robustness of the 3D system in inducing hematopoietic differentiation, we encapsulated the isogenic trisomic 21 (Tri21) iPSCs and subjected to hematopoietic differentiation and flow cytometry analysis as described above. As reported previously in 2D system (Banno et al., 2016, Cell Reports), Tri21 iPSCs showed higher percentage of hematopoietic commitment into erythroid and megakaryocytic lineages compared to disomic 21 (Di21), as characterized by the presence of respective surface markers (Fig 1F).
In conclusion, 3D culture system was suitable for inducing hematopoietic differentiation of iPSCs in isogenic disomic and trisomic lines. The 3D model can be used to generate patient-specific HSPCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.