Introduction
NCAPD3 (Non-SMC Condensin II Complex Subunit D3) is a regulatory subunit of the condensing-2 complex. Recent studies have shown that somatic mutations in genes encoding subunits of condensins associated with microcephaly, primary autosomal recessive and several cancers. NCAPD3 was highlighted as an outcome predictor in pancreatic ductal adenocarcinoma (PDAC) and a new biomarker for subtype-1 prostate cancer that improves prognostication. Yet, no literature has been reported regarding the expression and biological role of NCAPD3 in diffuse large B cell lymphoma (DLBCL). Hence, the aim of our study is to evaluate the functional significance and mechanism of NCAPD3 in DLBCL.
Methods
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers with informed consents. Expression levels of NCAPD3 mRNA and protein in DLBCL cell lines and PBMCs were detected by quantitative RT-PCR and western blotting. Immunohistochemistry (IHC) was conducted to assess the expression of NCAPD3 on paraffin-embedded tissues from 70 de novo DLBCL patients (study group) and 35 reactive hyperplasia patients (control group) with informed contents. Microarray datasets GSE32918 and GSE83632 were obtained from Gene Expression Omnibus. Survival analysis, protein-protein interaction (PPI) and gene set enrichment analysis (GSEA) in gene expression profiles were performed. Lentivirus vectors either targeting NCAPD3 (shNCAPD3) or empty lentiviral vector (shControl) were stably transfected into DLBCL cells. Cell proliferation was analyzed by cell counting kit (CCK-8). The apoptosis and cell cycle assays were carried out by flow cytometry. p < 0.05 was considered statistically significant.
Results
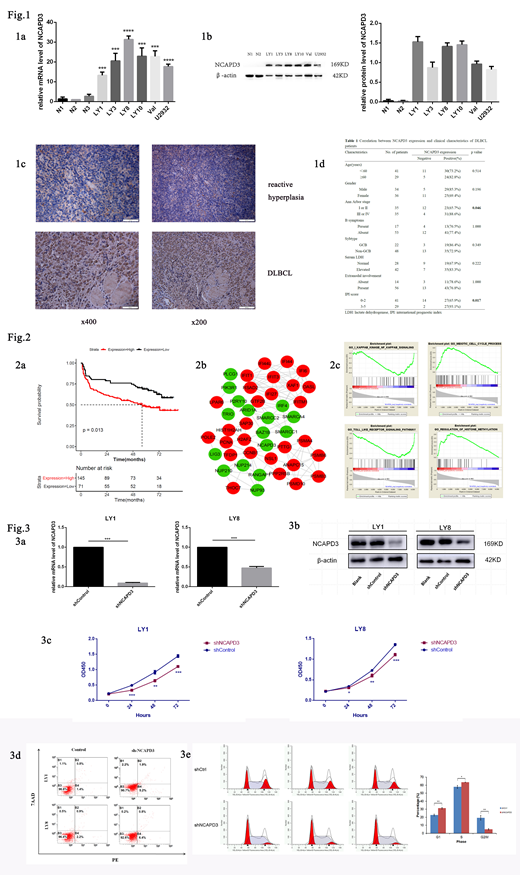
Markedly increased expression of NCAPD3 was detected in DLBCL cell lines at mRNA and protein level compared to those in healthy volunteers' PBMCs (Fig. 1a-b). We also observed higher NCAPD3 expression levels in DLBCL tissues than in reactive hyperplasia upon IHC staining (Fig. 1c). Expression of NCAPD3 protein was revealed in significant positive correlation with advanced Ann Arbor stage (p=0.046) and IPI score (p=0.017, Fig. 1d).
Bioinformatics analysis showed that high NCAPD3 expression in DLBCL was turned up to be correlate with shorter overall survival according to GSE32918 (p=0.013, Fig. 2a). PPI network and GSEA indicated that NCAPD3 was functional enriched in chromatin regulation, cell cycle, histone methylation, NF-κB signaling and Toll-like receptor signaling pathway (Fig. 2b-c). Relevant mechanism is now the focus of ongoing experiments.
Lentivirus mediated loss-of-function assays were performed to further investigate the biological role of NCAPD3 in DLBCL. Effective knockdown (shNCAPD3) was confirmed by qRT-PCR and western blot (Fig. 3a-b). Stable expression of shNCAPD3 in DLBCL cells exhibited growth suppression, increased fast-onset apoptosis, and induced GO/G1 phase arrest when compared to the control group (Fig. 3c-e).
Conclusion
Our investigations identified for the first time the oncogenic role of NCAPD3 in DLBCL tumorigenesis by bioinformatics analysis and in vitro evaluation. Expression of NCAPD3 was upregulated, and associated with adverse outcome of DLBCL patients. NCAPD3 inhibition by RNAi exerted anti-tumor efficacy in inhibiting cell growth, promoting apoptosis and blocking cell cycle. This study suggests that Sirt6 could be a potential molecular target for the treatment of DLBCL. Further study on it is under way.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.