Introduction:
There are limited options for acute myeloid leukemia (AML) patients who are too frail to receive intensive induction chemotherapy or who have relapsed /refractory disease. Early phase II trials published in 2016 demonstrated promising outcomes in AML with the BCL-2 inhibitor venetoclax, which was FDA approved in November 2018. We conducted a retrospective review of AML patients who received venetoclax at Kaiser Permanente Northern California (KPNC) specifically examining patients' characteristics and outcomes.
Methods:
This is a retrospective review of all KPNC patients who had a diagnosis of AML and received at least 1 prescription of venetoclax from January 1, 2016 through March 31, 2019. Data was abstracted from our electronic medical record. Variables included age, complete blood count, AML type, lines of therapy, prior use of hypomethylating agent, cytogenetics, somatic mutation, duration of treatment and chemotherapy regimen. Because none of the patients were treated under clinical trial protocol, most did not have a scheduled bone marrow biopsy to formally document disease status. Hematologic response (HR), defined here as neutrophil ≥ 0.5 x 109/L, platelet ≥ 50 x 109/L and RBC transfusion independence was used to access treatment effectiveness. Results were analyzed via descriptive statistics.
Results:
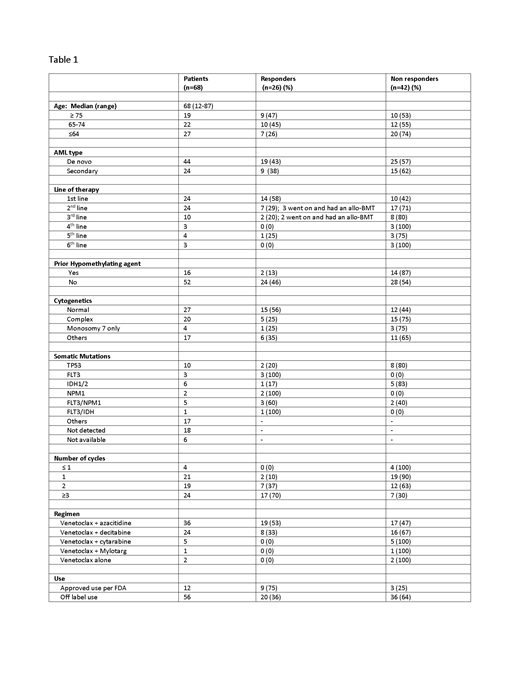
A total of 68 patients received venetoclax-based chemotherapy for treatment of AML. The median age was 68 (range 12-87). Among those 68 patients, 35% had venetoclax-based treatment as first line therapy, 35% as second line, and the remaining as third or later line (Table 1). Among those who received venetoclax as first line treatment, 58% had a HR. Among those who received venetoclax as second line treatment, 29% had a HR and 43% of those went on to allogeneic bone marrow transportation (BMT). 20% of those that received venetoclax in the third line setting had a HR and all went on to BMT. Patients who received venetoclax in fourth line or above did not have any significant response. Only 2 out of the 16 patients who received prior hypomethylating agent responded to venetoclax combination therapy. Four patients did not even complete cycle 1. 12 out of 68 patients were prescribed venetoclax based on FDA approved guidelines in November 2018. Of those 75% demonstrated HR.
Conclusion
While most of our patients used off-label venetoclax in a non-clinical trial setting, we confirm that venetoclax-based regimen is an effective treatment for AML, similar to published data. Our cohort represents a more heterogeneous population, which is more generalizable to the community. There is a higher response rate among those who used it in the first- and second-line setting (58% and 29% respectively). Venetoclax also showed efficacy in the second- or third-line setting, bridging patients to allogeneic BMT. Patients with prior hypomethylating agent are less likely to benefit from this drug. Venetoclax in combination with a hypomethylating agent is an effective AML regimen. Its efficacy deserves further studies, perhaps as a front-line treatment, sparing patients from intensive toxic inpatient induction chemotherapy.
No relevant conflicts of interest to declare.
This retrospective study evaluates the use of venenoclax-based regimen for treatment of AML. Clinicians have been using it since 2016, but the drug was not formally FDA approved for AML until November 2018.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract