Introduction: Isolated trisomy 11 is a rare, poorly characterized cytogenetic abnormality in myeloid neoplasms (MN) with only a few case series published. Prior reports suggested a lower response rate to standard treatments such as intensive chemotherapy (IC) and lower-intensity therapy (e.g. hypomethylating agents [HMA]), as well as higher relapse rates after allogeneic hematopoietic stem cell transplant (alloSCT) and poor overall survival (OS) [Table 1]. We sought to define the clinical and molecular characteristics of patients (pts) and their clinical outcomes.
Methods: We conducted a single center retrospective review of all adult pts with MN who were treated at Yale University from 1/1/2009 to 5/31/2019 and had trisomy 11 as the only cytogenetic abnormality. We collected data on age, sex, ethnicity, prior malignancies and their treatments, white blood cell (WBC), hemoglobin, platelet count, disease risk, initial and subsequent lines of therapies, and use of alloSCT. Responses were recorded using modified International Working Group (IWG) criteria 2003 for AML. OS was measured using Kaplan Meier methods. Molecular mutations were studied using a standard inhouse 40-gene next generation sequencing panel.
Results: We identified 11 pts with isolated trisomy 11 on initial presentation, of whom 10 had AML (de novo AML [8], AML with myelodysplasia-related changes [2]), while one had chronic myelomonocytic leukemia (CMML). Median age was 64 years (range [R] 43-78 years), 91% were male, and 91% were Caucasian (Table 2). There were no cases of therapy-related MN (t-MN) or myelodysplastic syndromes (MDS). Median WBC count on initial presentation was 19x109 /L (R: 1.2-232 x109) with a median of 42% peripheral blasts (R: 0-99%), and 2 pts presented with hyperleukocytosis (WBC >100 x109 /L). All cases of trisomy 11 on karyotype were also positive by fluorescence in-situ hybridization. Testing for somatic mutations was performed in 9 pts. Twenty somatic mutations were detected in 8 pts with the most common mutations affecting IDH2 (n=4 pts, 36.4%), IDH1 (n=3, 27.3%), DNMT3A (n=2, 18.2%), and U2AF1 (n=2; 18.2%). For initial therapy of the 10 AML pts, 6 pts were treated with intensive chemotherapy (IC) with 3 pts receiving 7+3 (one of whom previously received leukapheresis), one receiving high dose cytarabine, and 2 others receiving IC on clinical trials. Three pts were treated with HMA-based therapies, while the 10th pt received supportive care (SC) only. The CMML pt was treated on a clinical trial with an oral HMA.
Among AML pts, 6 pts achieved complete remission [CR] (including 5 of 6 pts treated with IC, and one out of 3 pts who received HMA), while the other 4 were primary refractory. Three pts relapsed during study follow-up. Of the 7 pts with relapsed/refractory (R/R)-AML, gemtuzumab ozogamicin + decitabine, an anti-CD47 antibody on trial, low-dose cytarabine + venetoclax (LDAC+VEN), and IC with cytarabine + mitoxantrone were used for 4 pts while the other 3 pts received only SC. Only one pt with R/R AML responded (he received LDAC + VEN) with a CR with incomplete count recovery (CRi). Most of these pts were treated before the approval of IDH inhibitors and therefore only one pt received an IDH inhibitor in second relapse.
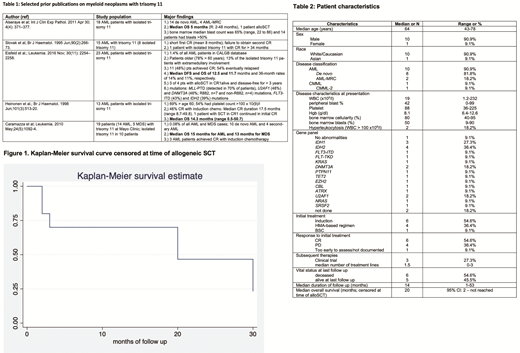
Six pts died during the study period while 5 were still alive at last follow up. Median duration of follow up was 14 months (R: 1-53 months). Three pts underwent alloSCT, all during first CR. Median OS of the entire cohort was 30 months (95%CI: 1.8 months - not reached). After censoring at the time of alloSCT, median OS was 20 months (95% CI: 2 months-not reached, [Figure 1]). A durable CR after alloSCT was observed in 1 pt (alive and in CR at 51 months after alloSCT), while another pt relapsed 8 months after alloSCT. A third pt remains in CR 8 months after alloSCT.
Interpretation: Here we report our single-center experience in MN pts with the very rare cytogenetic abnormality of trisomy 11. We confirm that this abnormality is extremely rare in MDS but can be seen in CMML. While in AML it is also rare, it does not seem to occur in the context of t-AML, and it tends to coexist with the targetable IDH-1/2 mutations. While we also confirm that survival is generally poor, OS among our pts appeared substantially longer than in prior studies with one pt being alive and in CR at 51 months after alloSCT.
Podoltsev:CTI Biopharma: Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sunesis Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Daiichi Sankyo: Research Funding; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Research Funding; Jazz Pharmaceuticals: Research Funding; Celgene: Other: Grant funding, Research Funding; Genentech: Research Funding; AI Therapeutics: Research Funding; Samus Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding; Kartos Therapeutics: Research Funding; Pfizer: Research Funding; Astex Pharmaceuticals: Research Funding. Gore:Celgene Corporation: Consultancy, Research Funding. Prebet:pfizer: Honoraria; novartis: Honoraria; pfizer: Honoraria; Agios: Consultancy, Research Funding; pfizer: Honoraria; pfizer: Honoraria; Bristol-Myers Squibb: Honoraria, Research Funding; pfizer: Honoraria; Boehringer Ingelheim: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; novartis: Honoraria; Tetraphase: Consultancy; Boehringer Ingelheim: Research Funding; novartis: Honoraria; novartis: Honoraria; Genentech: Consultancy; novartis: Honoraria; Boehringer Ingelheim: Research Funding. Isufi:Celgene: Consultancy; Novartis: Consultancy; Astra Zeneca: Consultancy. Foss:miRagen: Consultancy; Eisai: Consultancy; Seattle Genetics: Consultancy, Other: fees for non-CME/CE services ; Spectrum: Other: fees for non-CME/CE services ; Acrotech: Consultancy; Mallinckrodt: Consultancy. Huntington:Genentech: Consultancy; Pharmacyclics: Honoraria; Celgene: Consultancy, Research Funding; Bayer: Consultancy, Honoraria; DTRM Biopharm: Research Funding; AbbVie: Consultancy. Neparidze:Janssen Scientific Affairs, LLC: Research Funding; Eidos Therapeutics: Other: Member of Independent Diagnostic Committee; MMRF/Synteract: Membership on an entity's Board of Directors or advisory committees. Zeidan:Otsuka: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Research Funding; Celgene Corporation: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy, Honoraria, Research Funding; Medimmune/AstraZeneca: Research Funding; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Ariad: Honoraria; Agios: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Daiichi Sankyo: Honoraria; Cardinal Health: Honoraria; Seattle Genetics: Honoraria; BeyondSpring: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.