Background:
Ibrutinib is a Bruton's tyrosine kinase (BTK) inhibitor that was recently approved by the FDA for treatment of patients (pts) with relapsed/refractory marginal zone lymphoma (MZL) who have already received one or more anti-CD20 containing treatment regimens and require systemic therapy. Approval was based on a clinical trial in 60 pts that showed durable responses and a median progression-free survival (PFS) of 14.2 months (mos) (Noy et al., Blood 2017). Since pts outside clinical trials may have different disease and demographic characteristics, our study investigates the safety and efficacy of ibrutinib when implemented in a non-trial setting.
Materials and Methods:
This is a retrospective analysis of MZL pts who received ibrutinib monotherapy as part of their treatment at the University of Pennsylvania. Subjects were identified by a database search for any MZL pts prescribed ibrutinib. Primary endpoints were PFS and overall survival (OS) since initiation of ibrutinib. Secondary endpoints were overall response rate (OR) and complete response rate (CR), adverse events, and reasons for discontinuation. The first patient was treated on April 10, 2014, and the data cutoff was July 1, 2019. Analyses were performed using STATA 15.0 software.
Results:
There were 28 pts included in this study with a median age of 69 years (range 36-90) and median ECOG performance status at diagnosis of 0 (range 0-2). All pts had advanced disease (all stage III/IV & 68% with bone marrow involvement). The distribution of MZL subtypes was 43% extranodal, 25% nodal, and 32% splenic.
Most pts (89%) had received one or more treatments prior to ibrutinib (32% received first-line rituximab only). The median number of previous therapies was 2 (range 0-5), and 43% of pts were refractory to the previous line of therapy. A minority of pts (11%) received rituximab or another anti-CD20 antibody concurrently with ibrutinib. Pts started ibrutinib a median of 56 mos after their initial diagnosis (range 0.5-221 mos) with a median duration of therapy of 7 mos (range 0.7-62 mos). The median starting dose was 420 mg daily (range 70-560 mg daily).
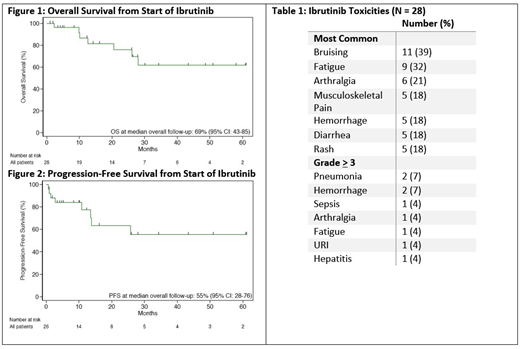
In 26 pts with response data available, the OR was 73% with CR 15%. The 12-mo PFS and OS were 77% and 87% respectively (see Figures 1 & 2, median PFS and OS not yet reached). PFS and OS at median follow-up were 55% and 69% respectively. There was no significant difference in response or survival rates among MZL subtypes. Pts who received rituximab only prior to ibrutinib had an OR of 86% compared to 69% in those with more than one previous therapy (Χ2 = 0.73, p = 0.39).
Ibrutinib was discontinued in 43% of pts after a median of 2.9 mos (range 0.7-13.7 mos) due to disease progression (50%), intolerance (42%), or other reasons (8%). Most (67%) pts subsequently received other therapies. All pts who stopped due to toxicity were responding at the time of discontinuation. Ibrutinib was also temporarily held or dose-reduced in 46% of pts due to toxicity (77%), preparation for surgery (15%), or drug interactions (8%). One patient experienced acute rebound of symptoms until ibrutinib was restarted.
Most commonly reported toxicities are summarized in Table 1. Grade 3 toxicities included pneumonia (7%), sepsis (4%), hemorrhage (4%), arthralgia (4%), fatigue (4%), URI (4%), and hepatitis (4%). Toxicities responsible for cessation of treatment were arthralgia, hepatitis, thyroiditis, and hemorrhage. There were no reported treatment-related deaths.
Conclusion:
To our knowledge, this is the first study to report the efficacy and safety of ibrutinib in a large cohort of MZL pts treated outside of a clinical trial. We found that the therapy was well-tolerated and observed response rates that compared favorably to those shown in the prospective clinical trial.
Hughes:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Genzyme: Membership on an entity's Board of Directors or advisory committees; Acerta Pharna/HOPA: Research Funding. Dwivedy Nasta:47 (Forty Seven): Research Funding; Rafael: Research Funding; Millenium/Takeda: Research Funding; Debiopharm: Research Funding; Aileron: Research Funding; ATARA: Research Funding; Pharmacyclics: Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Roche: Research Funding. Landsburg:Celgene: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Curis, INC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Curis, INC: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Speakers Bureau; Seattle Genetics: Speakers Bureau; Takeda: Research Funding; Takeda: Research Funding; Triphase: Research Funding; Triphase: Research Funding. Barta:Takeda: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Research Funding; Bayer: Consultancy, Research Funding; Mundipharma: Honoraria; Merck: Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Mundipharma: Honoraria. Chong:Novartis: Consultancy; Merck: Research Funding; Tessa: Consultancy. Schuster:Novartis, Celgene, Genentech, Merck, Pharmacyclics, Acerta, and Gilead: Other: Grants, Research Funding; Novartis, Nordic Nanovector, and Pfizer: Membership on an entity's Board of Directors or advisory committees; Nordic Nanovector, Pfizer, AstraZeneca, Loxo Oncology, Acerta, and Celgene: Honoraria; Novartis: Other: a patent (with royalties paid to Novartis) on combination therapies of CAR and PD-1 inhibitors.. Svoboda:AstraZeneca: Consultancy; Celgene: Research Funding; Incyte: Research Funding; Pharmacyclics: Consultancy, Research Funding; Kyowa: Consultancy; Merck: Research Funding; BMS: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding.
This paper looked at the effectiveness of ibrutinib, a Bruton's tyrosine kinase inhibitor, in treating patients with marginal zone lymphoma (MZL). Ibrutinib is FDA approved for MZL patients who have received at least one anti-CD20 containing therapy and now require systemic treatment. Some patients in the study had not previously received an anti-CD20-based therapy and received ibrutinib off-label.
Author notes
Asterisk with author names denotes non-ASH members.