INTRODUCTION
While chemoimmunotherapy is effective in indolent B-cell non-Hodgkin lymphoma (iBCL), most patients are older and may benefit from a chemotherapy-free approach. Pembrolizumab is an immune checkpoint inhibitor which blocks the PD-1 receptor. Upon chronic antigen stimulation, T-cells can lose their anti-tumor activity due to PD-1 signaling. Multiple in vitro and in vivo studies have demonstrated that blockade of PD-1 can reverse the T-cell dysfunction induced by the tumor microenvironment. Pembrolizumab has shown an excellent safety and toxicity profile.
Pembrolizumab as a single agent is FDA approved for numerous solid tumors and some lymphoma subtypes including Hodgkin lymphoma and primary mediastinal B-cell lymphoma. PD-1 inhibitors have also shown activity in the relapsed/refractory iBCL with overall responses reported to range from 10 to 60% (Ding et al., 2017; Lesokhin et al., 2016; Nastoupil et al., 2017a). However, the overwhelming majority of the patients in these studies had received prior chemotherapy. Patients with untreated iBCL have a relatively intact immune system and may have a greater capacity to mount an effective anti-tumor immune response upon reversal of T-cell dysfunction.
OBJECTIVES
The primary objective is evaluate the efficacy of pembrolizumab as monotherapy for patients with untreated iBCL based on ORR measured at the end of a 6-cycle treatment period.
Secondary objective include:
Safety and toxicity.
Efficacy including complete response, clinical benefit rate (complete response + partial response + stable disease) ≥6 months, time to next therapy, progression-free survival, and duration of response.
SUBJECTS AND METHODS
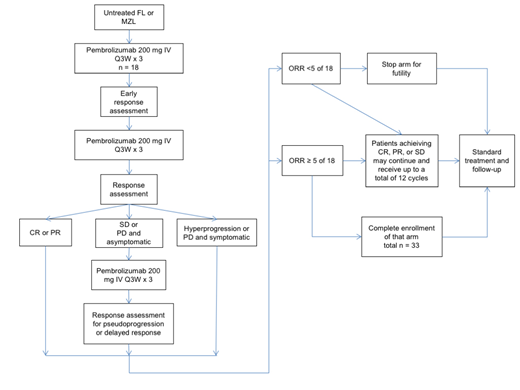
Patients ≥ 18 years of age with previously untreated radiographically measurable follicular or marginal zone lymphoma with an indication for treatment are included. These indications include significant symptoms, end organ damage, cytopenias, or steady progression. Major exclusions include known autoimmune disease, major organ dysfunction, or ECOG ≥ 2. Treatment consists of 200 mg pembrolizumab IV on day 1 of each 21-day cycle. Initial response assessment occurs after 6 cycles. Patients with a complete or partial response can remain on the trial. Patients who have stable disease or progressive disease and are asymptomatic (excluding hyperprogression) will receive 3 additional cycles and then will be restaged to account for a delayed response. Those patients with a complete or partial response after those additional cycles can remain on the trial. Subsequently, response assessment occurs every 3 cycles. Patients without progressive disease or unacceptable toxicity are able to continue up to 18 cycles. Up to 33 patients will be enrolled, and follow-up may continue for an additional 2 years.
STATISTICAL METHODS
The primary efficacy endpoint is the overall response rate (CR + PR). Secondary endpoints include duration of response, progression free survival, and time to next therapy (see treatment schema). A sample size of 33 participants is planned to provide 80% power at the 5% significance level to distinguish the observed overall response rate from a true rate of ≥ 40% versus ≤ 20%) using a Simon 2-stage minimax design. The trial will be terminated early if fewer than 5 of the first 18 patients have a response. The thresholds used in this design are based on what is considered to be a clinically meaningful response rate with a novel agent with very low rates of adverse events and toxicity compared to standard regimens. This response rate threshold is also higher than what has been observed using anti-PD-1 therapy in the relapsed/refractory setting.
RECRUITMENT
Three patients have been accrued to the study. To date, all patients have remained on study with no grade 3-5 adverse events.
CORRELATIVE STUDIES
Given that pembrolizumab is known to modulate the tumor immune microenvironment, we are also performing clinical correlates on a companion protocol. Using several 22 color flow cytometry panels and 6-color multiplex immunohistochemistry, we will analyze pre- and post-treatment lymph nodes and peripheral blood. These studies will examine over 50 populations of immune cells in addition to T-cell activation/exhaustion markers such as PD-1, TIM-3, LAG-3, CTLA-4, and PD-L1.
TRIAL REGISTRATION
Clinicaltrials.gov (NCT03498612). Funding for both the trial and correlative studies has been provided by Merck.
Gopal:Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte.: Consultancy; Seattle Genetics, Pfizer, Janssen, Gilead, Sanofi, Spectrum, Amgen, Aptevo, BRIM bio, Acerta, I-Mab-pharma, Takeda, Compliment, Asana Bio, and Incyte: Honoraria; Teva, Bristol-Myers Squibb, Merck, Takeda, Seattle Genetics, Pfizer, Janssen, Takeda, and Effector: Research Funding. Lynch:Rhizen Pharmaceuticals S.A: Research Funding; Johnson Graffe Keay Moniz & Wick LLP: Consultancy; T.G. Therapeutics: Research Funding; Takeda Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Juno Therapeutics: Research Funding. Ujjani:Pharmacyclics: Honoraria; PCYC: Research Funding; Astrazeneca: Consultancy; Atara: Consultancy; Gilead: Consultancy; Genentech: Honoraria; AbbVie: Honoraria, Research Funding. Shadman:ADC Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Sound Biologics: Consultancy; Mustang Bio: Research Funding; TG Therapeutic: Research Funding; Pharmacyclics: Consultancy, Research Funding; Verastem: Consultancy; Gilead: Consultancy, Research Funding; Celgene: Research Funding; AbbVie: Consultancy, Research Funding; Acerta Pharma: Research Funding; BeiGene: Research Funding; Sunesis: Research Funding; Astra Zeneca: Consultancy; Atara Biotherapeutics: Consultancy. Smith:Denovo Biopharma: Research Funding; Incyte Corporation: Research Funding; Pharmacyclics: Research Funding; Genentech: Research Funding; Ignyta (spouse): Research Funding; Merck Sharp & Dohme Corp: Consultancy, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta Pharma BV: Research Funding; Ayala (spouse): Research Funding; Bristol-Myers Squibb (spouse): Research Funding; Seattle Genetics: Research Funding; Portola Pharmaceuticals: Research Funding. Till:Mustang Bio: Patents & Royalties, Research Funding. Fromm:Merck, Inc.: Research Funding. Shustov:Seattle Genetics, Inc.: Research Funding.
Pembrolizumab for the treatment of indolent non-Hodgkin lymphoma
Author notes
Asterisk with author names denotes non-ASH members.