Background: Central nervous system (CNS) involvement by peripheral T cell lymphoma (PTCL) is a rare condition. Among primary CNS lymphomas, only 2% are secondary to PTCL, while the risk of CNS relapse in all cases of PTCL is estimated at 2% to 6%. Little is known about the presentation and outcomes of PTCL patients with CNS involvement given the rarity of this entity. In this study, we describe patient characteristics, histology, and clinical course of patients with CNS involvement by PTCL.
Methods: The Mayo Clinic Lymphoma Database was used to identify PTCL patients with primary or secondary CNS involvement seen at our institution between 2000 and 2018. A total of 12 patients were identified and their medical records were reviewed for patient and disease characteristics, CNS-directed treatment modality, and outcomes. The Kaplan-Meier method was used for time-to-event analysis.
Results: The median age at CNS diagnosis was 63 years (range 41 to 76) and 11 (93%) patients were male. The histological diagnoses were PTCL, NOS in 9 (75%) patients, enteropathy-associated T-cell lymphoma in 2 (17%) patients, and angioimmunoblastic T-cell lymphoma in 1 (8%) patient. Five patients presented with primary T-cell CNS lymphoma (all with a PTCL, NOS histology), while the remaining 7 (58%) patients also had systemic involvement.
All patients presented with neurologic symptoms at the time of CNS involvement diagnosis including: focal motor deficits in 6 patients (unilateral upper extremity weakness, gait impairments, and hemiparesis), cognitive decline in 5 patients (memory impairments, reduced attention, and confusion), headache in 4 patients, and seizure in 3 patients. The CNS disease location included the brain parenchyma in 9 (75%) patients, leptomeninges in 1 (8%) patient, and lumbar plexus in 1 (8%) patient. One patient (8%) had positive CSF finding only without radiologic evidence of involvement.
CSF analysis was performed in 11 patients. Elevated protein levels were noted in 3 (27%) patients, malignant cells in 2 (18%), and no clear abnormalities in the remaining 6 (55%) patients. Concomitant bone marrow involvement was seen in only 1 patient. Elevated LDH was seen in 2 patients. The a median LDH was 195 U/L (range 139 to 4,360)
The most common CNS-directed therapies were: high-dose methotrexate (MTX)-based regimens in 8 (67%) patients, including high-dose MTX in combination with temozolomide (n=2), or cytarabine and thiotepa (n=2). Intrathecal MTX, temozolomide and dexamethasone, lenalidomide, high-dose steroids, and surgical resection were the treatment modality used for one patient each.
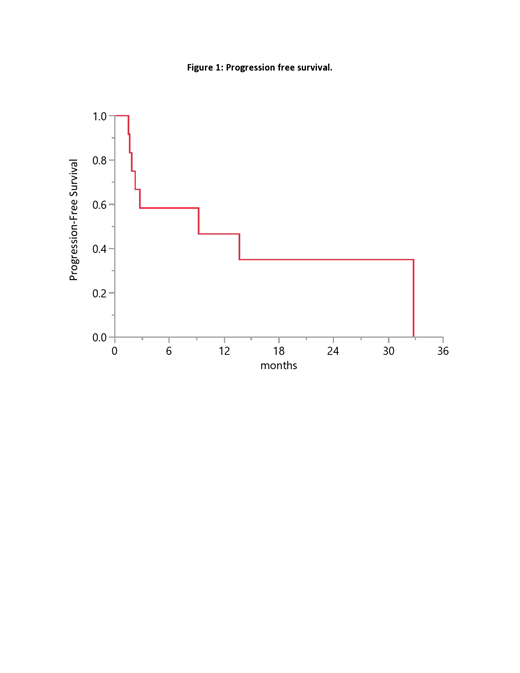
At a median follow up of 18 months, eight (75%) out of 12 patients were not alive at the time of last follow up. The median overall survival (OS) from diagnosis was 16 months (95% CI: 2.8-173). The median progression free survival (PFS) from initiation of CNS-directed therapy was 9 months (95% CI: 1.6-33) (figure). Four patients had a PFS longer than 12 months. These 4 patients were treated with: temozolomide/dexamethasone, high-dose MTX, lenalidomide, and high-dose MTX followed by cytarabine/thiothepa.
Conclusion:
CNS involvement by T-cell lymphoma is a rare complication that carries a poor prognosis. Early onset of neurologic symptoms should trigger prompt investigation of CNS involvement. Despite the short OS and PFS, some patients may achieve a relatively longer disease free interval.
Bennani:Adicet Bio: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Seattle Genetics: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Adicet Bio: Other: Advisory board; Purdue Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board; Kite Pharma: Other: Advisory board. Cerhan:Celgene: Research Funding; NanoString: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Nowakowski:Celgene: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Curis: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Genentech, Inc.: Research Funding; MorphoSys: Consultancy, Research Funding; NanoString: Research Funding; Selvita: Membership on an entity's Board of Directors or advisory committees. Ansell:Mayo Clinic Rochester: Employment; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding. Paludo:Celgene: Research Funding; Verily Life Sciences: Research Funding; Verily Life Sciences: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.