Background:Apolizumab (APO) is a humanized IgG1 antibody targeting a HLA-DRβ chain specific epitope, which is expressed by approx. 50 % of B cell lymphomas. The antibody's clinical development was hampered by toxicity, potentially mediated by its strong CDC activity. However, previous studies demonstrated that APO efficiently triggered tumor cell killing by myeloid cells, especially by granulocytes. Myeloid effector cell engagement is strongly regulated by CD47-SIRPα interactions, and blockade of this axis has been demonstrated to improve the efficacy of therapeutic antibodies. Hence, our goal was to characterize the influence of CD47-SIRPα blockade on myeloid cell engagement by APO.
Methods: Expression of the APO epitope, CD20 and CD47 on different B lymphoma cell lines was measured by flow cytometry. The capacity of APO and Rituximab (RTX) to induce complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC) by mononuclear (MNC) or polymorphonuclear (PMN) effector cells was analysed by 51Cr release assays. Antibody-dependent cellular phagocytosis (ADCP) by monocyte-derived macrophages was analysed by fluorescence microscopy. PMN-mediated ADCC and macrophage-mediated ADCP experiments were performed in the presence or absence of the CD47 blocking antibody 5F9-IgG2σ to analyse the impact of CD47-SIRPα blockade on APO-mediated lymphoma cell killing by myeloid cells.
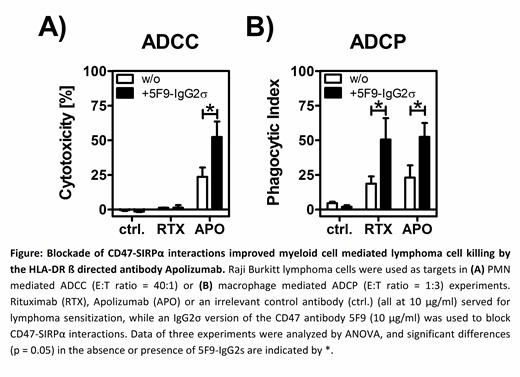
Results: As anticipated we observed binding of APO on approx. 50% of the tested B lymphoma cell lines, including Raji (Burkitt's lymphoma), MINO (mantle cell lymphoma) and Carnaval (double hit DLBCL). Focusing on these cell lines, APO's CDC activity was more potent (Raji) or similar (Mino) compared to RTX. Furthermore, APO significantly induced tumor cell lysis by PMN or MNC in 51Cr release assays and achieved higher lysis rates in comparison to RTX. Blocking CD47-SIRPα interactions by 5F9-IgG2σ resulted in significantly increased APO-induced ADCC by PMN against all tested B cell lymphoma lines (Mino: 28.8 ± 10.2 % vs. 41.8 ± 13.3 %; Carnaval: 37.9 ± 17.9 % vs. 55.6 ± 17.6 %; Raji: 23.6 ± 11.7 % vs. 52.4 ± 19.4 % for APO alone vs. APO + 5F9-IgG2σ, respectively, p < 0.05 by ANOVA). However, no ADCC by PMN and RTX was observed - even in the presence of CD47-SIRPα blockade. Moreover, APO mediated similar or even stronger ADCP by macrophages compared to RTX, which could be further enhanced by 5F9-IgG2σ (Figure 1).
Conclusions: APO's potent myeloid cell activation could be further enhanced by disruption of CD47-SIRPα interactions. Our observations suggest to generate isotype variants of APO with lower CDC but preserved or enhanced ADCC activity. Subsequently, a combination of APO and CD47-SIRPα blocking strategies may improve B cell lymphoma immunotherapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.