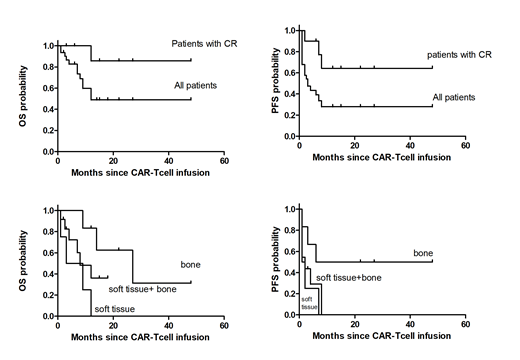
Factors associated with complete remission and durable remission after CD19 chimeric antigen receptor (CAR)-modified T-cell immunotherapy for aggressive B-cell non-Hodgkin lymphoma (NHL) have not been identified. We report multivariable analyses of factors affecting response and progression-free survival (PFS) in patients with aggressive NHL treated with CD19 CAR T cells. The best overall response rate was 71%, with 32% of patients achieving complete remission. The median PFS and OS of patients with aggressive NHL who achieved complete remission were 11 months and 12 months. The median PFS and OS of patients with bone and bone marrow involvement without soft tissue involvement were 14 months and 18 months. The median PFS and OS of patients with soft tissue involvement were 1 months and 4 months. We report complete remission rates and long-term survival rates in patients with bone and bone marrow involvement were significantly higher than those in patients with soft tissue involvement.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.