BACKGROUND: Axi-cel is an autologous anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that is approved for treatment of relapsed/refractory (R/R) large B-cell lymphoma and is associated with high response rates and durable remissions. Recent data show that axi-cel is effective across various adverse prognostic features, namely cell of origin, disease bulkiness, and extranodal disease, among others. Hypoalbuminemia is a known adverse prognostic factor in lymphomas. It is unknown if axi-cel overcomes the adverse prognostic feature of hypoalbuminemia in R/R large B-cell or transformed follicular lymphoma.
METHODS: We conducted a retrospective analysis of patients treated with axi-cel across three Mayo Clinic campuses (Rochester, Jacksonville, and Phoenix) from 06/01/2018 until 04/01/2019. The primary objective of this analysis was to assess the impact of hypoalbuminemia (defined at day 0, prior to infusion) on outcome after axi-cel therapy.
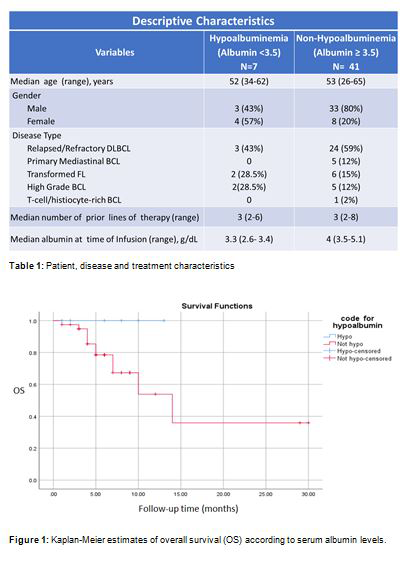
RESULTS: A total of 50 (male=37, 74%) patients (pts), median age of 53 (26-67) years received axi-cel. The median number of prior lines of therapy was 3 (2-8) (Table 1). Two pts had no available serum albumin levels at time of axi-cel infusion. Seven (15%) of 48 pts had serum albumin levels lower than 3.5 g/dL (median= 3.3 g/dL (range 2.6-3.4)) and the median follow up of survivors was 7.6 (1.9-14.3) months. The best overall response rate (ORR) and complete remission (CR) rates in these pts were 57% and 57%, respectively. One (14%) patient had stable disease and 2 (29%) had disease progression. The median overall survival (OS) for pts with hypoalbuminemia was not reached. On the other hand, 41 (85%) pts had a normal serum albumin level (median=4.0 (range 3.5-5.1) g/dL) and the median follow up for survivors was 6.3 months. The best objective response rate (ORR) and complete remission (CR) rates in these pts were 82% and 44%, respectively. The median OS for pts with normal serum albumin was 14 (95%CI=6.3-29.6) months. There was no significant difference at 6-months and 1-year OS between pts with hypoalbuminemia vs. those with normal baseline serum albumin levels [6-month=100% vs. 79%(95%CI=64-93%); 1-year (100% vs. 54% (95%CI=26-82%), p=0.17] (Figure 1).
All grades cytokine release syndrome (CRS) was diagnosed in all 7 pts with hypoalbuminemia (100%) and in 38 of 41 (92%) pts without hypoalbuminemia. There was no difference in the median duration of CRS between pts with or without hypoalbuminemia [6 (range 1-11) days vs 5 (range 1-19) days, p=0.89]. Neurotoxicity (all grades) was observed in 5 (71%) pts with hypoalbuminemia compared 26 (63%) with normal albumin levels. There was no statistically significant difference in median duration of neurotoxicity between pts with hypoalbuminemia and those with normal baseline albumin levels [9 (range 1-10) days vs. 3 (range 0-25) days, p= 0.72].
CONCLUSIONS: Hypoalbuminemia does not have a significant impact on the outcomes of axi-cel therapy, including the incidence of CRS or neurotoxicity. These results need to be validated in a large collaborative multicenter study. Further investigation is needed to assess the prognostic impact of severe hypoalbuminemia (<3g/dL) on axi-cel therapy.
Ansell:Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Affimed: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Trillium: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Trillium: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Trillium: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Seattle Genetics: Research Funding; Affimed: Research Funding; Affimed: Research Funding. Bennani:Seattle Genetics: Other: Advisory board; Kite Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Adicet Bio: Other: Advisory board; Seattle Genetics: Other: Advisory board; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board; Purdue Pharma: Other: Advisory board; Purdue Pharma: Other: Advisory board; Adicet Bio: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Kite Pharma: Other: Advisory board. Paludo:Verily Life Sciences: Research Funding; Celgene: Research Funding; Verily Life Sciences: Research Funding; Celgene: Research Funding. Tun:DTRM Biopharma: Research Funding; Mundi-pharma: Research Funding; BMS: Research Funding; Celgene: Research Funding; Curis: Research Funding; TG Therapeutics: Research Funding. Foran:Agios: Honoraria, Research Funding. Kharfan-Dabaja:Daiichi Sankyo: Consultancy; Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.