Introduction
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm frequently associated with debilitating disease-related symptoms and shortened overall survival (OS). Ruxolitinib, a Janus kinase (JAK)1/JAK2 inhibitor, was approved on November 16, 2011 for patients with intermediate- and high-risk MF. The phase 3 COMFORT trials demonstrated that ruxolitinib reduced spleen volume, improved MF-related symptoms, and prolonged OS. In the COMFORT trials, the median duration of ruxolitinib treatment was 2.8 years (5-year pooled analysis). The objective of this real-world chart review study is to describe persistence of ruxolitinib treatment early on in the treatment course in patients with MF at community hematology/oncology practices in the United States.
Methods
Medical charts from community hematology/oncology practices in Cardinal Health Oncology Provider Extended Network (OPEN) were reviewed and abstracted into electronic case report forms by physicians. Physicans were instructed to abstract data from the earliest eligible patients and then chronologically, thereafter. This analysis included adult patients diagnosed with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF between 01/2012 and 12/2016. Patients were required to have intermediate- or high-risk MF (based on a data-derived International Prognostic Scoring System [IPSS] risk categorization), have platelet count >50 × 109/L at the time of diagnosis, have received ruxolitinib as first-line treatment, and have ≥6 months of follow up after the initiation of ruxolitinib; patients who died within 6 months of initiation of ruxolitinib were considered as discontinued patients and were included in the analysis. Three- and 6-month persistence rates are reported.
Results
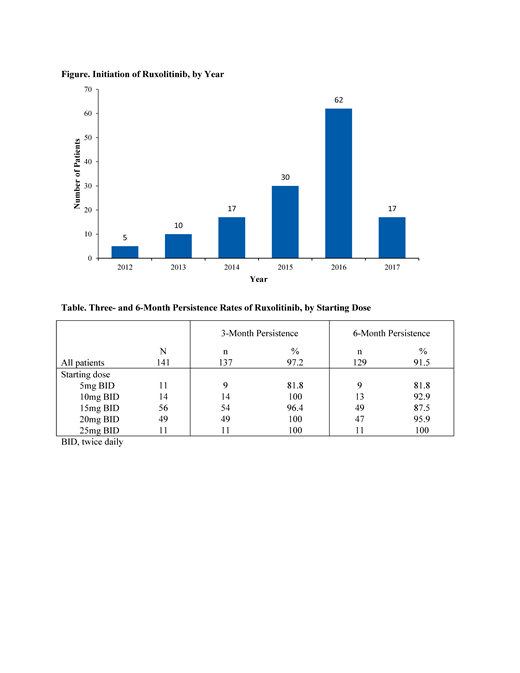
A total of 141 patients from 22 community hematology/oncology practices were included in the analysis; 3 patients who were still on ruxolitinib at the end of the study period, but with less than 6 months of follow up, were excluded from the analysis. Mean (SD) age at diagnosis was 69.8 (10.2) years, 61.7% were male, 71.6% had primary MF, and 56.7% were high-risk at diagnosis. Median time from diagnosis to ruxolitinib initiation was 36 (interquartile range [IQR], 20-91) days. The majority of patients initiated ruxolitinib in 2016 or later (Figure). The starting dose of ruxolitinib was 5 mg twice daily [BID] in 7.8%, 10 mg BID in 9.9%, 15 mg BID in 39.7%, 20 mg BID in 34.8%, and 25 mg BID in 7.8%. Three- and 6-month persistence rates were 97.2% and 91.5%, respectively (Table).
Discussion
A previous retrospective chart review of prescription claims and patient level data evaluated a cohort of patients with MF prescribed ruxolitinib just prior to and shortly after its approval (November 1, 2011 to August 31, 2012); in that study, 3- and 6-month persistence rates of 39.1% and 27.0%, respectively, were reported (Blood. 2013[122]:2833). In the current study, the 3- and 6-month persistence rates were notably higher at 97.2% and 91.5%, respectively. These higher rates are likely attributable to a number of factors. The current analysis was limited to patients treated first-line with ruxolitinib. Furthermore, the index period in the current analysis extended almost 5 years beyond ruxolitinib approval; in this 5-year period, there were a number of publications in support of ruxolitinib dose optimization with particular focus on the first 8-12 weeks of treatment (J Hematol Oncol. 2013[6]:79; Int J Hematol. 2016[104]4:420-9). The 3- and 6-month persistence rates reported here demonstrate that only a small proportion of patients with myelofibrosis treated with first-line ruxolitinib discontinued the drug early on in their treatment course.
Conclusion
This contemporary analysis demonstrates high rates of 3- and 6-month persistence of first-line ruxolitinib in patients with MF treated in community practices. Proper monitoring and management of the expected mechanism-based toxicity of a JAK inhibitor and proper dose titration for insufficient response, early in the treatment course, may help patients derive maximum, long-term benefits from ruxolitinib.
Yu:Incyte: Employment, Equity Ownership. Kish:Cardinal Health: Employment. Paranagama:Incyte: Employment, Equity Ownership. Colucci:Incyte: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.