Introduction:
The various chimeric antigen receptor (CAR) genes used in most CAR-T clinical trials are expressed via the EF1α promoter. Recent reports have suggested that CAR expression using the myeloproliferative sarcoma virus enhancer, with the negative control region deleted and the dl587 Rev-binding site substituted (MND) promoter, to drive CAR expression results in a higher efficiency of gene transduction. The use of a different promoter for CAR expression is expected to alter the expression levels and the surface densities of CAR in transduced T cells. It has been reported previously that while only a few ligands are required to activate TCR signaling, CAR-T cells require approximately 100 CD19 molecules to trigger cytotoxic killing. Here we generated two CAR-T vectors (1904A and 1904B) using respectively the EF1α and the MND promoters to drive gene expression and compared them in cell culture and in a preclinical study.
Methods:
The second-generation CD19-CAR with T2A-linked tEGFR was expressed by using the EF1α or the MND promoter. The expression levels of tEGFR and CAR were determined by flow cytometry using the biotinylated-Erbitux, and the CD19-Fc protein and the α-FMC63scFv antibody, respectively. The in vitro cytotoxicity assay was performed by co-culture of CAR-T and Calcein-AM-stained K562-CD19. NOD-SCID mice engrafted with Raji-luciferase cells were used as an animal model to validate the activities of 1904A and 1904B CAR-T. A randomized, double-blinded, and dual-arms prospective clinical study was registered (NCT03840317) to compare the safety and efficacy of the treatments for recurrent/refractory (r/r) B-ALL. Subjects were assigned in two groups: those with blasts (by morphology) in bone marrow between 5-20% or above 20%. All subjects were preconditioned with fludarabine and cyclophosphamide before 3x105 CAR-T cells/kg infusion.
Results:
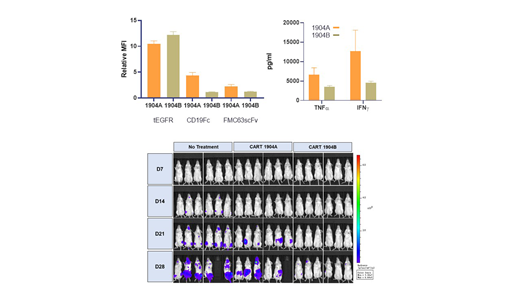
While tEGFR expression in 1904A and 1904B CAR-T cells was similar (with relative MFIs of 10.47±1.16 and 12.19±1.30 respectively), a significant increase in CAR expression was detected in 1904A compared to 1904B (with relative MFIs of 2.26±0.37 vs 1.24±0.07 and 4.37±0.59 vs 1.13±0.08 as determined by α-FMC63scFv and CD19Fc staining), indicating increased CAR expression on the surface of 1904A CAR-T cells. Importantly, 1904A cells showed greater cytotoxicity and release of cytokines, especially IFNγ and TNFα in cell culture. Both 1904A and 1904B treatments prolonged the median survival time in the animal model. Interestingly, the increase of tumor cells in mice was slower in the group treated with 1904B, suggesting 1904B T cells were more susceptible to the inhibitory effect of the tumor. In the clinical study, only 6 and 8 subjects for 1904A and 1904B respectively completed the trial by the end of July 2019. The median follow-up for 1904A and 1904B were 127 (67-168) and 138.5 (45-179) days, and tumor burdens were 3.06% (0.68%-82.7%) and 4.49% (1.29%-39.0%), respectively. The adverse events of subjects were monitored and recorded according to CTCAE 5.0. No significant difference in disease incidence or severity was detected between the two groups. The CR rate is 83.3% (5/6) for the 1904A group, and 100% (8/8) for the 1904B group. There were 3 (1904A) and 5 (1904B) subjects who bridged to HSCT. All subjects remained in remission with one CD19-negative recurrence in the 1904B group.
Conclusion:
Using the myeloproliferative sarcoma virus enhancer, we have generated a lentivirus vector, 1904B that can express CAR at a lower level on the cell surface, and investigated 1904B CAR-T functions in cell culture, in a mouse model, and in patients. Lower surface expression of CAR was found with reduced cytotoxicity and lower cytokine release of CAR-T in cell culture, but prolonged T cell activity in a mouse model. Human subjects treated with 1904B CAR-T developed fever later and shorter than 1904A, with slower expansion of CAR-T cells. We suggest that a lower surface expression of CAR molecules can decelerate the kinetics and decrease the intensity of CAR-T activation in vitro and in vivo.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.