Background
Anabolic steroids, such as danazol, have been used for years in the treatment of myelodysplastic syndromes (MDS). Their successful use has been documented in aplastic anemia, hypoplastic MDS, and immune thrombocytopenia, among other hematologic conditions. In patients with low-risk MDS, available treatment options are limited. Danazol is frequently used to improve symptoms and modify the disease course; however, its efficacy and optimal dosing schedule are unclear.
Methods
We conducted a systematic review of the literature to identify studies that used anabolic steroids, including danazol, in the treatment of MDS. In accordance to guidelines, our systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under ID CRD42019121467. We included case series with more than 15 patients, observational studies (cohorts and case control), and randomized trials where anabolic steroids were used to treat MDS. Following PRISMA guidelines, 2 independent reviewers assessed the studies obtained through the search strategy. Discrepancies were resolved by a third reviewer. Due to the heterogeneity of the findings, no meta-analysis was performed.
Results
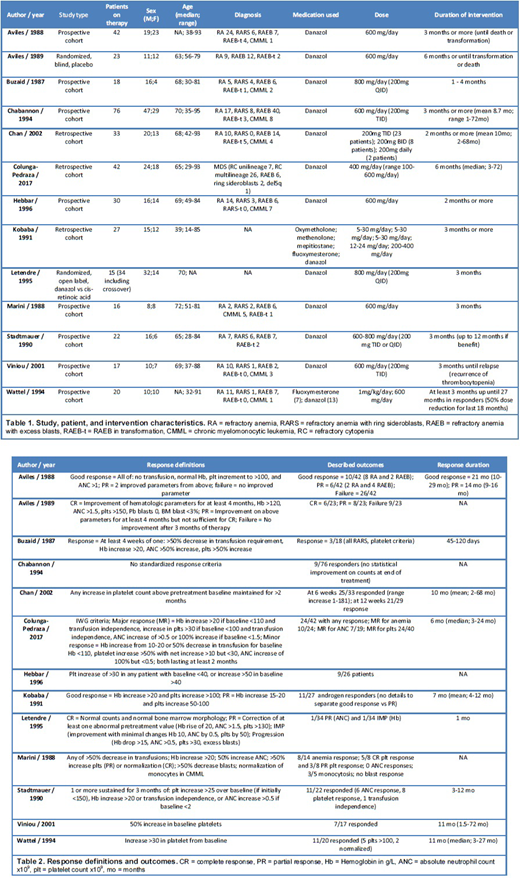
A total of 1000 studies were identified through our search. After removing duplicates and undergoing Level 1 screening by 2 reviewers, 69 full text articles were assessed. Thirteen studies were included in the final review as the other studies did not meet inclusion criteria. Of these, 3 were retrospective cohorts, 8 prospective cohorts, and 2 randomized trials. A total of 366 patients with MDS were exposed to anabolic steroids throughout the studies with diverse outcomes reported. FAB classification was used by most of the studies to categorize MDS, with one exception that used the 2008 WHO classification. Diagnoses included refractory anemia, refractory anemia with ring sideroblasts, refractory anemia with excess blasts (RAEB), RAEB in transformation, and chronic myelomonocytic leukemia. All but 2 studies used danazol as their intervention, with the exceptions using other anabolic steroids as well as danazol. Dosage varied across studies with the majority aiming for a dose of danazol 600mg daily. Patient characteristics and interventions are summarized in Table 1. The intervention was sustained for at least 1 month and up to 12 months across studies. Outcomes reported are diverse with positive and negative studies identified. Unfortunately, outcomes are not comparable due to the presence of different response definitions with different hematologic improvement cutoffs. Reports of response were highly variable, with studies showing 5 - 75% response rates depending on their study response definitions. Multiple studies reported a trend in platelet count improvement. Response duration was not specified by all studies and was highly variable across the reports. Response definitions and outcomes are shown in Table 2. No significant adverse effects to danazol were reported in any of the studies reviewed. No specific analysis of benefit throughout the different MDS groups was reported in any of the studies.
Conclusion
Our systematic review highlights the significant lack of data and irregular results across studies. The heterogeneity of included populations, MDS classification, response criteria, and interventions, do not allow for evidence based recommendations regarding the use of danazol or anabolic steroids in MDS patients. Given the limited treatment options for low-risk MDS patients, particularly in low and middle income countries, further well designed studies are needed.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.