INTRODUCTION
The diagnosis of CMML according to WHO 2017 requires the presence of ≥1x109/L and ≥10% of monocytes in peripheral blood (PB). Establishing an accurate diagnostic is difficult since many clinical situations present persistent monocytosis. The presence of dysplasia is frequent but not always present and cytogenetic aberrations are infrequent in this disease (20-25% of cases). Although 85-90% of CMML patients present ≥1 mutation in TET2, SRSF2 or ASXL1, the use of NGS panels is not widespread. The study of PB monocyte subsets by flow cytometry (FC) has gained interest for CMML diagnosis. The increase of classical monocytes (Mo1) upper 94% presents a high sensitivity (Sn) and specificity (Sp) for CMML diagnosis (Sn 90.6, Sp 95.1; Selimoglu-Buet et al, Blood 2015). The 94% threshold was validated in two studies (Talati C et al, Blood 2017; Tarfi S et al, Blood Cancer J 2018). However, some controversies have recently appeared in the literature. Picot T detected the 95% cutoff as the one with the best Sn (100%) and Sp (97%) (Picot T et al, Front Oncol 2018). Hudson CA found that the presence of < 1.13% (Sn 100, Sp 96) of non-classical monocytes (Mo3) was the best predictor for CMML diagnosis (Hudson CA et al, Am J Clin Pathol 2018). With the exception of the study of Tarfi S, based on 47 CMML, the rest presented a very low number of patients (Talati C: 29; Picot T: 15; Hudson CA: 16) and therefore a bias could be expected specially when studying the Sn of the proposed methods. Moreover, the different series assessing the "monocyte assay" have no molecular data and therefore this could diminish the accuracy of the results since some patients may have received misdiagnoses.
The aim of our study was to assess the Sn and Sp of different thresholds of Mo1 and Mo3 in a large series with well-annotated clinical, cytogenetic and molecular data. Moreover, we assessed whether the study of CD2 and CD56 monocyte expression in combination with the %Mo1 >94 test improves the detection of the disease.
METHODS
50 CMML, 12 MDS, 11 MPN with ≥1x109/L monocytes and 79 reactive monocytosis with ≥1x109/L monocytes (N = 152) were prospectively studied from 02/2016 to 07/2019. We studied PB monocyte subsets by FC: Mo1 (CD14bright/CD16-), Mo2 (CD14bright/CD16+) and Mo3 (CD14dim or -/CD16bright). In addition, we assessed the expression of CD56 and CD2 in monocytes (positivity ≥ 20%). Finally, targeted NGS of the entire exonic sequence of 25 genes recurrently mutated in myeloid malignancies was performed (VAF sensitivity: 2%). Chi-Square or Fisher exact tests were used as appropriate. ROC curves were developed to explore optimal cutoffs in terms of sensitivity (Sn) and specificity (Sp). Moreover, we plotted the AUC of the subset of Mo1 and Mo3. Finally, the Youden index (YI) was used to detect the threshold of Mo1 and Mo3 with the best balance between Sn and Sp.
RESULTS AND DISCUSSION
The Sn and Sp of the Mo1>94% test in our series were similar to those reported by the French group (GFM). Our Sn and Sp were 90% and 92% respectively with a YI of 82. The Sn and Sp of the Mo1>93% were 94% and 84% with a YI of 78. Finally, the 95% cutoff proposed by Picot T et al showed a Sn of 81% and a Sp of 96% with a YI of 77. Therefore, the 94% cutoff presented the best balance between Sn and SP of the different thresholds assessed.
The Mo3 threshold of 1.13% proposed by Hudson CA et al showed a Sn of 67% and a Sp of 95% with a YI of 62. The best Mo3 cutoff in our series was established in 3.18% with a Sn of 90% and Sp of 83%. The YI of this threshold was 73.
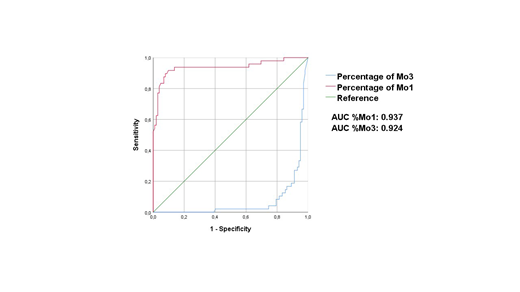
The AUC for the percentage (%) of Mo1 (0.937, IC 95%: 0.89-0.99) was better than the AUC of the % of Mo3 (0.924, IC 95%: 0.88-0.97) reinforcing the use of %Mo1 as the item with the best discriminative power for CMML diagnosis. The AUC of the percentage of Mo1 population was similar to that reported by the GFM (Figure 1).
The Sn and Sp for CD56 expression in monocytes was 67% and 91% respectively, while CD2 expression showed a Sn of 38% and a Sp of 99%.
Finally, the presence of at least one of the following: Mo1 >94%, CD56+ or CD2+ presented the highest Sn (98%) and a Sp of 84%. This method may be a very good screening test due to the low false negative rate expected. This combined approach showed the best balance between Sn and Sp (YI: 82).
CONCLUSIONS
Our study supports the utility of the Mo1 >94% test as the best flow cytometry assay for establishing accurate diagnoses in CMML. The combined assay of Mo1, CD56 and CD2 may be of high utility as a screening test.
Bellosillo:Qiagen: Consultancy, Speakers Bureau; TermoFisher Scientific: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.