Introduction
Bispecific T-cell engager (BiTE) antibodies represent a novel therapeutic option for patients with multiple myeloma (MM). BiTE antibodies lack Fc region, and have variable domain only, they can simultaneously bind to two different epitopes i.e. cluster of differentiation 3 (CD3) molecules on tumor-specific T cells, and a specific antigen on myeloma cells, which leads to T-cell dependent destruction of myeloma cells. Currently, blinatumomab, specific for CD3 and CD19 is the only Food and Drug Administration FDA approved BiTE antibody for clinical use in patients with relapsed/refractory (RR) B-cell acute lymphoblastic leukemia, several similar BiTE antibodies are under development.
Methods
Following PRISMA guidelines, we performed comprehensive literature on 4/15/19 cross-referencing the terms "bispecific antibodies" and "multiple myeloma" using PubMed, Embase, Web of Science, Cochrane Library, Clinicaltrials.gov and review of international medical meeting abstracts. Initially, 256 articles were identified and after detailed scrutiny, one phase 1 clinical trial with prelim results, 4 preclinical and 4 ongoing clinical trials were included.
Results
Preclinical trials:
Anti-BCMA x Anti-CD3 Bispecific Antibody: BiTE antibodies are still in early development in MM, and most of the published data is about the pre-clinical phase. In preclinical trials, Hipp et al. 2017 and Cho et al. 2018 reported that AMG 420 (BI 836909) and AMG 701, which are anti CD3 and B-cell maturation antigen (BCMA), are highly efficacious in vitro in the killing of myeloma cells and potently induces autologous tumor cell lysis in patients with both newly diagnosed and RRMM regardless of their disease status. In mouse xenograft models reconstituted with human T cells, in vivo efficacy of AMG 420 was reported with an overall response in 6 of 10 animals, with all 6 responders became tumor-free at the end of the study. In an orthotopic L-363 xenograft model, treatment with AMG 420 resulted in prolonged median survival of 43-43.5 days. Dilillo et al. 2018 and Ji Li et al. 2017 reported similar in vivo results for REGN5458 and BFCR4350A respectively.
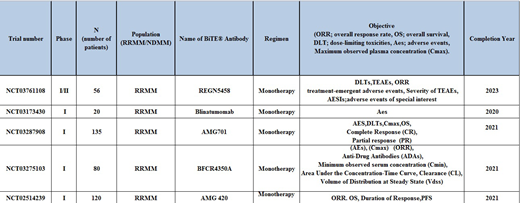
Clinical trials:
Currently, there are 5 phase 1 ongoing clinical trials (Table 1). Updated results of only first in human phase I AMG 420 are available. Forty-two MM patients with a high tumor burden and four prior lines of therapy were given 2.5 treatment cycles with AMG 420. Overall thirteen (31%) patients responded to AMG 420 therapy, with complete response (CR) in 6 (14.2%) patients, very good partial response (VGPR) in 2 (5%) patients and partial response (PR) in 2(5%) patients. Eleven of these patients responded in the first treatment cycle, with a median response time of 1 month. Twenty-five (57.1%) patients discontinued treatment due to progressive disease. Four deaths were reported; 2 from disease progression and 2 due to adverse events; neither of them was treatment-related. Serious adverse events were reported in twenty-one (50%) patients, the infection was reported in twelve (29%) and polyneuropathy in three (7%), eighteen (43%) required hospitalization. Treatment-related serious adverse events included three (7%) patients with grade 2-3 cytokine release syndrome, three (7%) with polyneuropathy and one (2.3%) with edema.
Conclusion
After the success of naked antibodies like daratumumab and elotuzumab for MM, there is a need to develop immunotherapy using conjugated antibodies and BiTE antibodies to overcome the challenge of MM resistance and relapse to prior therapies. Preclinical data with BiTE antibodies are promising; AMG 420 anti-CD3/BCMA BiTE has already been granted fast track status by the FDA. We anticipate that drug will enter phase 2 clinical trials for drug development against RRMM Other BiTE antibodies with strong preclinical efficacy are under development and data from larger prospective clinical trials is needed to explore their efficacy in the treatment of multiple myeloma.
Anwer:In-Cyte: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.